Protein in situ expression chip, and constructing method and application thereof
A protein and chip technology, applied in biological testing, material inspection products, etc., can solve the problems of template molecules and capture molecules that cannot be fixed in an orderly and gradual manner, spatial conformation changes, and loss of activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the three-dimensional protein chip of detecting the content of hepatitis B virus core antibody (HBc Ab), hepatitis B virus e antibody (HBe Ab) and hepatitis B virus surface antibody (HBs Ab) in the body of hepatitis B patient
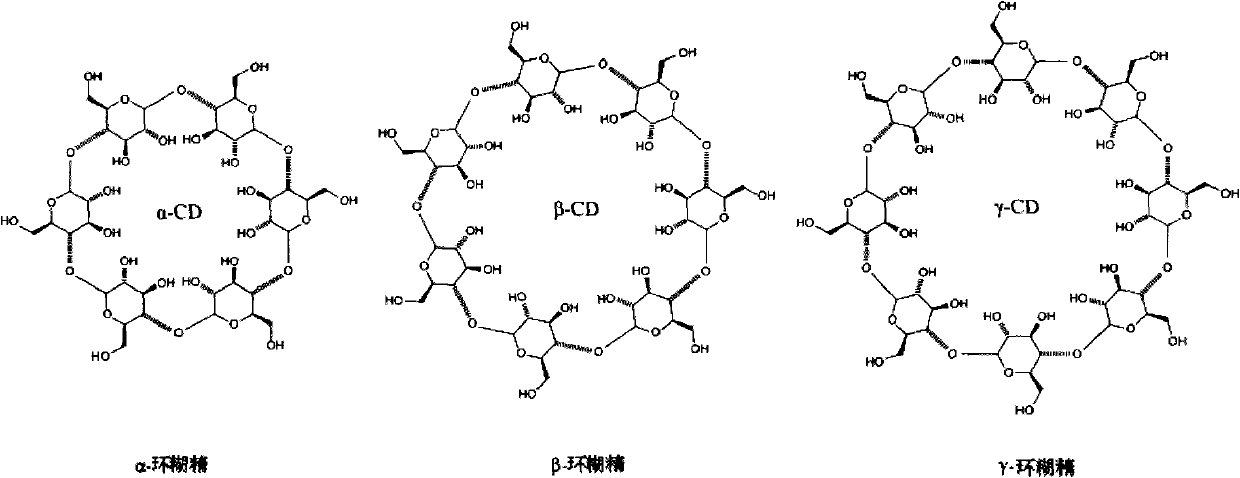
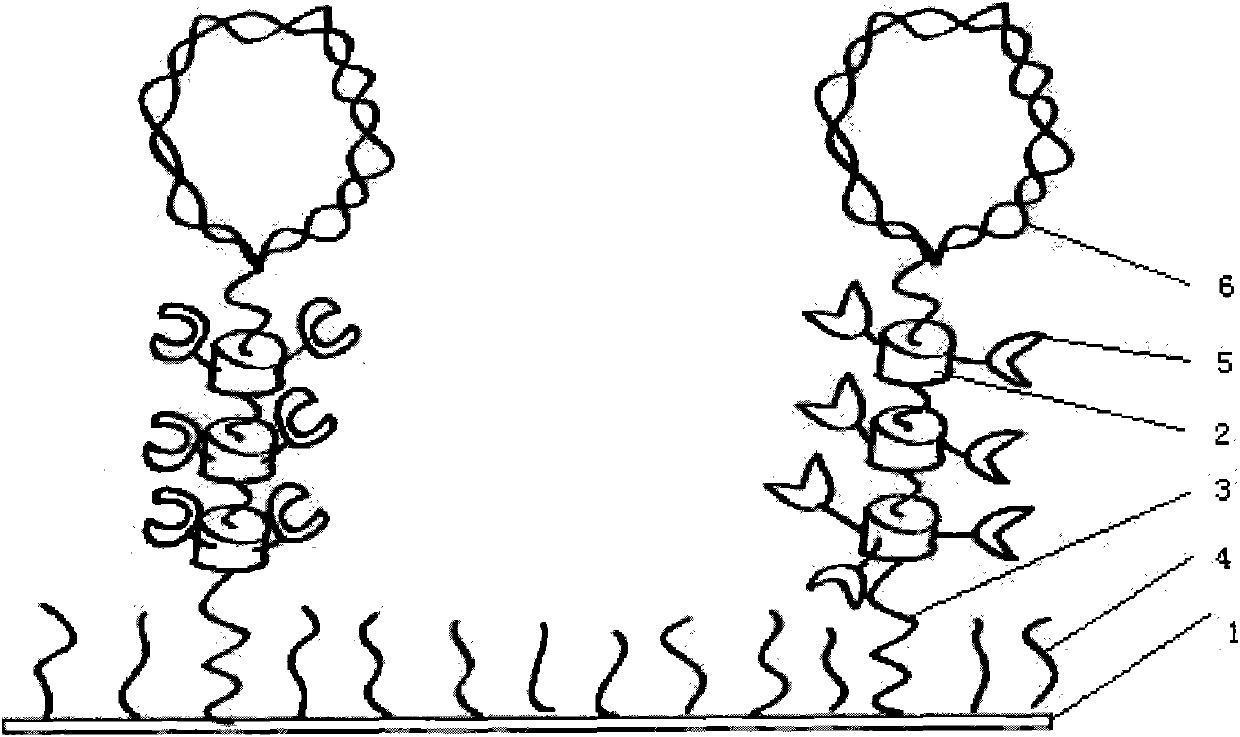
[0094] Evaporate 2nm of chromium and 40nm of gold on the surface of a glass slide with a high refractive index (the glass slide is 75mm*25mm), and modify the surface with long and short chain PEG molecules with mercapto groups, in which the first chain molecule PEG 45 ends at a carboxyl group , the second chain molecule PEG 6 terminal is a hydroxyl group, mixed at a ratio of 1:10, self-assembled on the gold surface, the total concentration is 1mM, the dosage is 100ul, and evenly spread on the surface of the gold sheet. After that, add Ni-NTA-modified α-cyclodextrin molecular solution (concentration: 100ug / ml, 40ul), and let it stand for 1 day, so that the cyclodextrin molecules self-assemble on the first chain molecule PEG to form ...
Embodiment 2
[0095] Example 2: Anti-protein diffusion chip containing four characteristic proteins of p21, p31, gp41 and gp120
[0096] On the gold-coated glass substrate, the surface is modified with long and short chain molecule dithiol molecules with sulfhydryl groups, in which the end of the first chain molecule dithiol (Mw: 8000) is a carboxyl group, and the second chain molecule Molecular dithiol Mw: 1500 terminal is a hydroxyl group, the structural formula is as follows. Among them, the first chain molecule R is a carboxyl group, and n is 45; the second chain molecule R is a hydroxyl group, and n is 6:
[0097]
[0098] Mixed at a ratio of 1:50, self-assembled onto the gold surface at a total concentration of 1 mM. After that, add 50ug / ml, 50ul Ni-NTA-modified α-cyclodextrin molecular solution, and let it stand for 1 day, so that the cyclodextrin molecules self-assemble on the first chain molecule dithiol to form a polyrotaxane structure . After using NHS / EDC solution to act...
Embodiment 3
[0099] Example 3: Discovery of Biomarkers for Autoimmune Diseases
[0100] 2nm of chromium and 40nm of gold are vapor-deposited on the surface of the glass slide with a high refractive index, and the surface is modified with long and short chain PEG molecules with mercapto groups, in which the end of the first chain molecule PEG45 is a carboxyl group, and the second chain molecule PEG6 The terminal is a hydroxyl group, mixed at a ratio of 1:10, self-assembled on the gold surface, the total concentration is 1mM, and the dosage is 100μl, and evenly spread on the surface of the gold sheet. Afterwards, a saturated concentration of α-carboxymethylcyclodextrin molecular solution (125mg / ml, 50ul) was added and allowed to stand for 1 day to allow the cyclodextrin molecules to self-assemble on the first chain molecule PEG to form a polyrotaxane structure. After using NHS / EDC solution to activate the carboxyl group on the first chain molecule PEG 45 and carboxymethyl cyclodextrin, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com