Compositions comprising tramadol and celecoxib in the treatment of pain
A technology of celecoxib and its composition, which is applied in the field of treating severe to moderate pain, can solve problems such as the undetermined correlation of ulcer healing, and achieve good blood-brain barrier penetration and pain treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
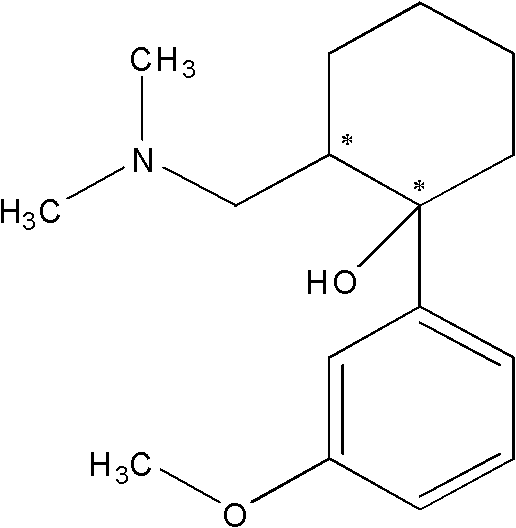
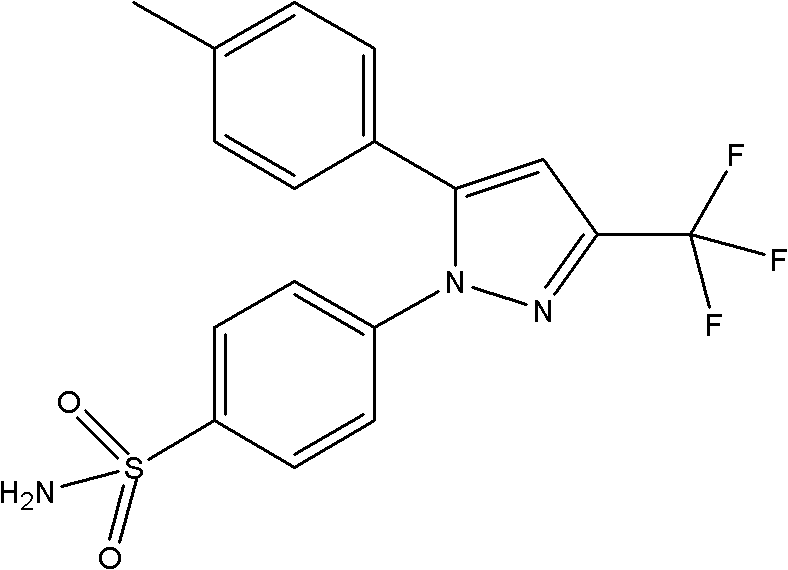
[0069] Preparation of composition doses of tramadol hydrochloride and celecoxib
[0070] Preparation of racemic tramadol hydrochloride and celecoxib at different molar ratios (1:1, 1:3 and 3:1) of (racemic)-tramadol·HCl:celecoxib combination. All drugs and combinations were dissolved in distilled water containing 0.5% hydroxypropylmethylcellulose and administered at 10 ml / kg per rat via intraperitoneal (i.p.) route. Table 1 lists the different ratios prepared at various concentrations.
[0071] Table 1. Corresponding doses of each drug or combination administered intraperitoneally
[0072]
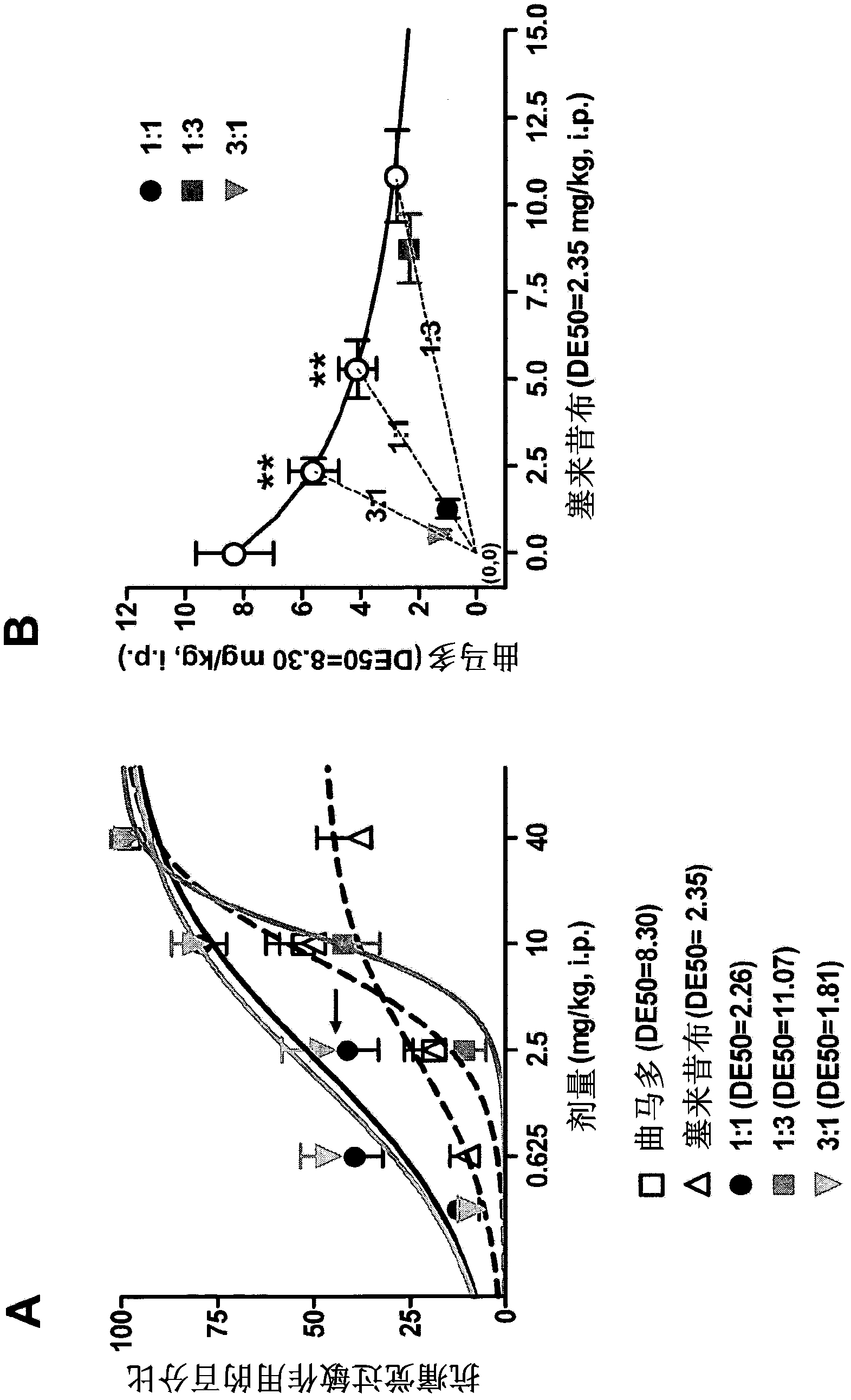
[0073] Effects of thermal hyperalgesia in a postoperative pain model in rats
[0074] The aim of this study was to evaluate the elimination of the combination comprising tramadol / celecoxib, in particular at different molar ratios (1:1, 1:3 and 3:1), in a model of postoperative pain following paw incision in rats. Analgesic efficacy and potency of a combination of tramadol hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com