Process for production of cytokine-induced killer cells
A cytokine and cell killing technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problems of low proliferation of CIK cells and large physical burden, and achieve the effect of high therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 "CIK cell culture using anti-human CD3 antibody and Retronectin
[0082] (1) Separation and preservation of PBMC
[0083] From healthy people with informed consent, Donor A, Donor B, and Donor C, separate blood samples were taken (the so-called blood samples in this article are blood collection for the purpose of collecting monocytes). Dulbecco PBS (manufactured by Invitrogen or Nissui Pharmaceutical Co., Ltd.; hereinafter referred to as DPBS) or physiological saline containing 1% human serum albumin (preparation name: ブミネート; manufactured by Bectstar, hereinafter referred to as HSA) (Hereinafter referred to as 1% HSA / normal saline) Dilute the obtained components by about 2 times, and put them on Ficoll-Paque PREMIUM or Ficoll-Paque PLUS 15mL (both are produced by GE Healthcare Biosciences) The diluted components were collected and separated into 30 mL of blood, and centrifuged at 700×g for 20 minutes at room temperature. After centrifugation, the PBMC layer was r...
Embodiment 2
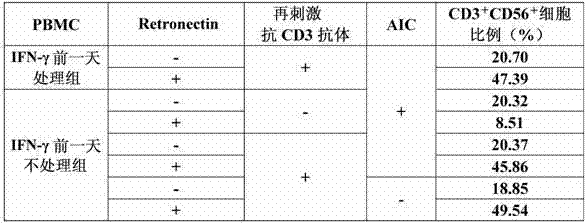
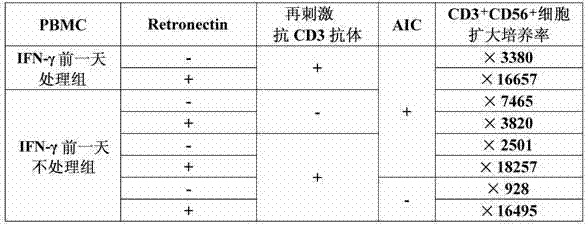
[0108] Example 2 "Culturing CIK cells using anti-human CD3 antibody and Retronectin (comparison of adding IFN-γ and using auto-irradiated cells)
[0109] (1) Preparation of autologous irradiated cells (hereinafter referred to as AIC)
[0110] After suspending the donor A-derived PBMC prepared in Example 1-(1) in 0.5% HAB / 0.2% HSA / GT-T503, an X-ray irradiation device was used to irradiate 3400R (29.8Gy) X-rays (hereinafter The cells after X-ray irradiation are called PBMC AIC). Press 1.06×10 of the prepared PBMC AIC 6 Cells / mL were resuspended in 0.5% HAB / 0.2% HSA / GT-T503.
[0111] (2) Cultivation of CIK cells
[0112] PBMC derived from Donor A prepared in Example 1-(1) was calculated as 1.06×10 6 Cells / mL were suspended in 0.5% HAB / 0.2% HSA / GT-T503 (referred to as the IFN-γ group without treatment the day before). However, a group was also established in which the same prepared PBMC was cultured overnight in the presence of IFN-γ (final concentration 1000 U / mL) on the day before the...
Embodiment 3
[0128] Example 3 "Culturing CIK cells with anti-human CD3 antibody and Retronectin (comparison of anti-human CD3 antibody concentration during restimulation)
[0129] (1) Preparation of PBMC AIC
[0130] Except for using donor B-derived PBMC or donor C-derived PBMC instead of donor A-derived PBMC, PBMC AIC was prepared in the same manner as in Example 2-(1).
[0131] (2) Cultivation of CIK cells
[0132] CIK cells were cultured in the same manner as in Example 2-(2). However, PBMC from Donor B or PBMC from Donor C (using the same donor-derived PBMC as the donor used in Example 3-(1)) was used for PBMC, and no IFN-γ treatment was performed the day before. In addition, when the anti-human CD3 antibody was re-stimulated on the 8th day of culture, the concentration of the anti-human CD3 antibody was 50 ng / mL or 200 ng / mL.
[0133] Continue the culture until the 14th day of the start of the culture. On the 14th day after the start of the culture, measure the number of viable cells by the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com