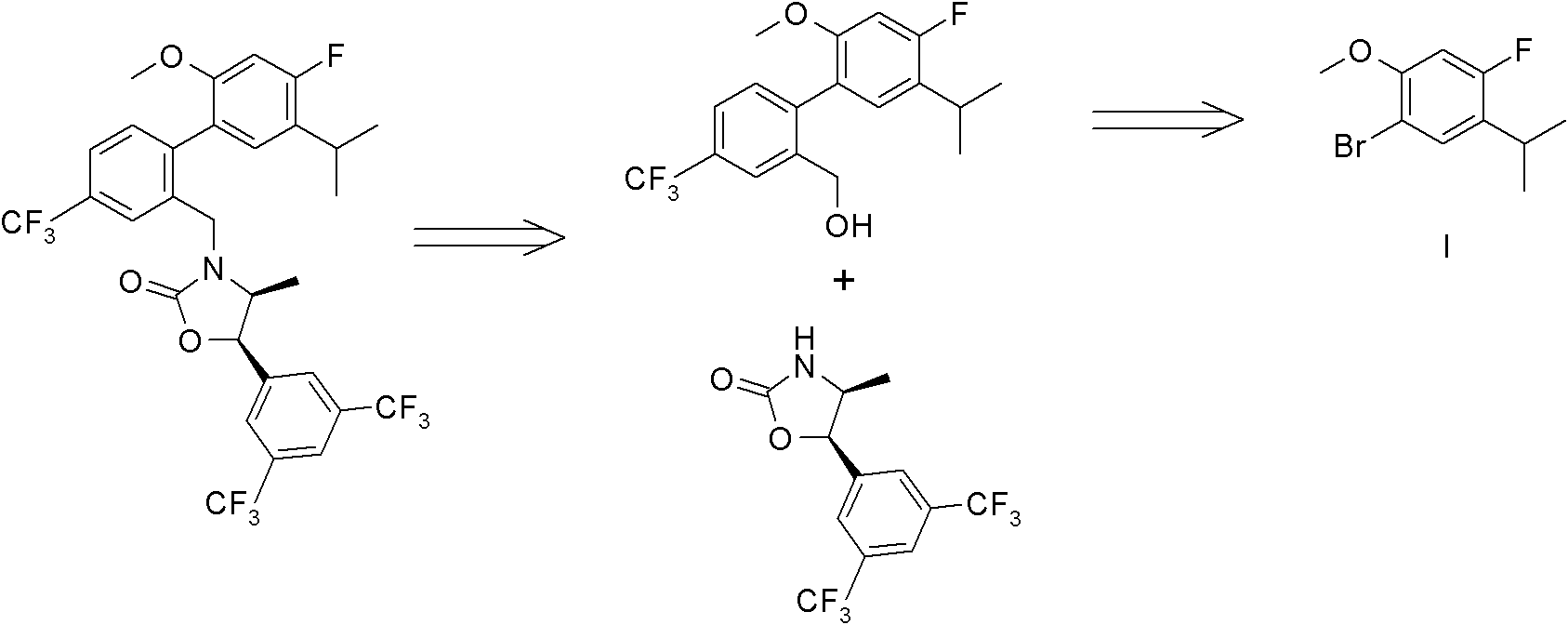
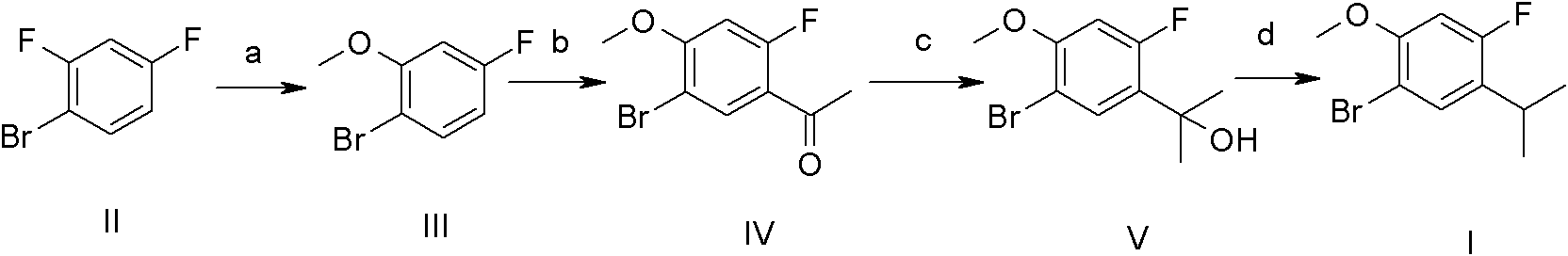
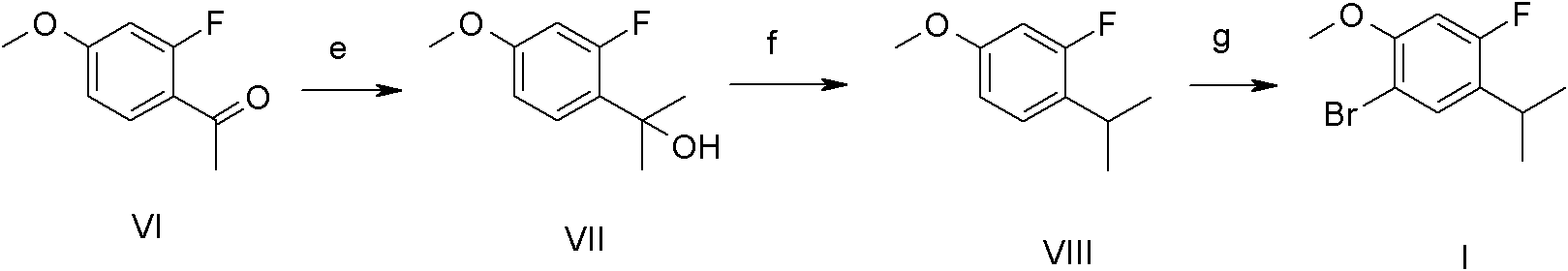
Synthetic method of 1-bromo-4-fluorin-5-isopropyl-2-metoxybenzene
A technology of tetramethyldisiloxane and compound is applied in the field of synthesis of 1-bromo-4-fluoro-5-isopropyl-2-methoxybenzene, and can solve the problems of expensive raw materials, low yield, heavy metal Pollution and other problems, to achieve the effect of mild reaction conditions, high product purity, and simple processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Compound VI:
[0032]
[0033] AlCl 3 (1000g, 7.500mol, 1.5eq) was added to 1,2-dichloroethane (DCE) (5000mL), and compound IX (630.0g, 5.000mol, 1.0eq) was added slowly under an ice-water bath. After the addition, Ac at 0~5℃ 2 O (510.5 g, 5.000 mol, 1.0 eq). Then the mixture was reacted at room temperature for 3 h. The reaction solution was poured into 5000 g of ice, the organic phase was separated, and then the organic phase was washed with 8% NaOH aqueous solution (5000 mL), and then washed with 5000 mL of saturated brine. Anhydrous Na 2 SO 4 Dry and concentrate. The crude product was recrystallized from petroleum ether (PE) to obtain 708.1 g of compound VI as a white solid, yield: 85%. 1 H NMR (400M Hz, CDCl3) δ (ppm) 7.90 (t, J = 8.80Hz, 1H), 6.76 (dd, J = 2.36, 8.84Hz, 1H), 6.63 (dd, J = 2.36, 13.08Hz, 1H ), 3.88 (s, 3H), 2.61 (d, J=5.20Hz, 1H).
[0034] Synthesis of Compound VII:
[0035]
[0036] Add magnesium chips (112.4g, 4.625mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com