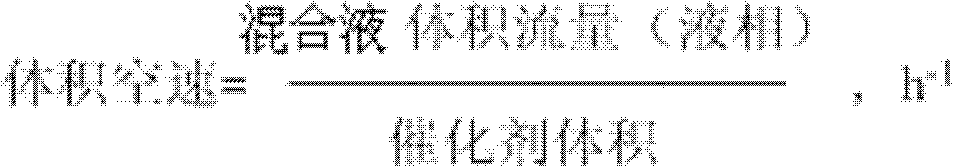Synthesis method of triethylamine and catalyst used in method
A triethylamine and catalyst technology, applied in the field of organic compound synthesis, can solve the problems of difficult separation of triethylamine, complex reaction process, poor product quality, etc., and achieve low price, simple operation, and good atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
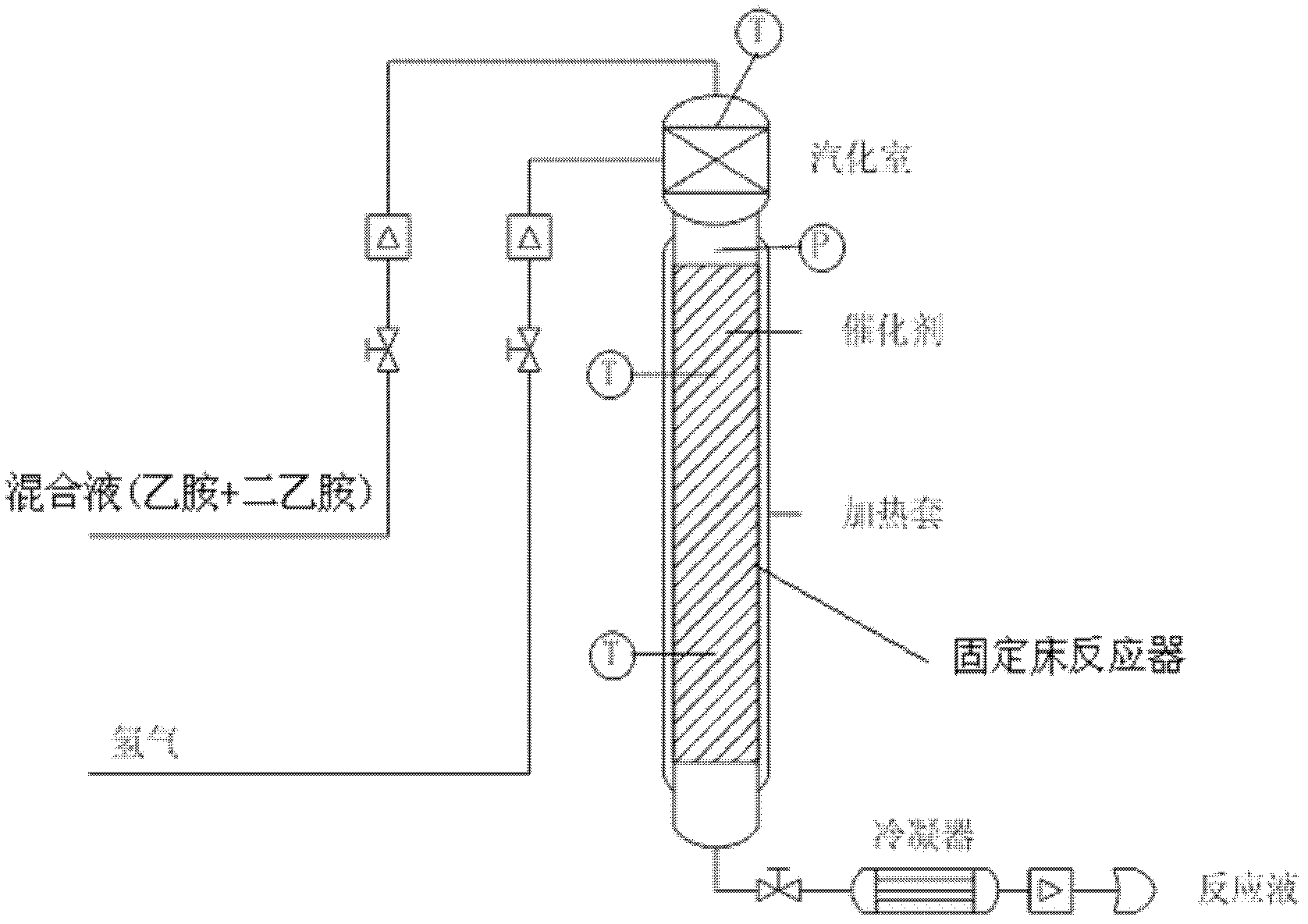
[0038] Embodiment 1, a kind of production method that ethylamine and diethylamine disproportionation reaction synthesizes triethylamine, carry out according to the following steps successively:
[0039] 1), preparation of nickel-copper-palladium catalyst (i.e., a supported catalyst for the synthesis of triethylamine);
[0040] a), commercially available γ-Al with a particle diameter of 2 to 3 mm 2 o 3 First roast at 240-260°C for 3 hours, and then roast at 290-310°C for 3 hours; get roasted γ-alumina. After testing: the specific surface area of the γ-alumina after roasting is 230-250m 3 / g, the pore size is 10-11.5nm; the particle diameter remains unchanged.
[0041] b), the roasted γ-Al obtained in step a) 2 o 3 Immerse in water for 36 hours, and then measure the volume of water reduction, so as to obtain the γ-Al after calcination 2 o 3 The pore volume density is 0.75ml / g;
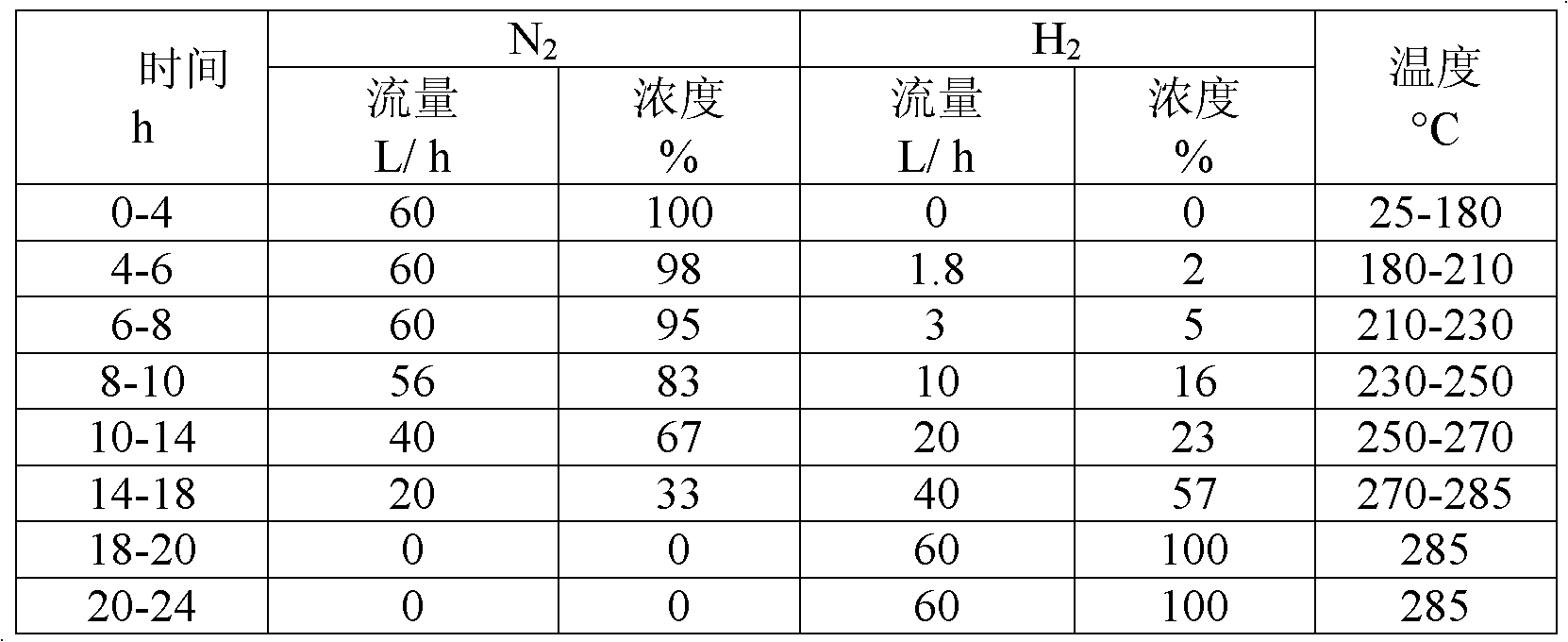
[0042]c), with 46.71g nickel nitrate solid (not containing crystal water, hereinafter the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com