Method and composition for color modulation
A composition, a technology of a cosmetic composition, applied in the field of skin tone regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
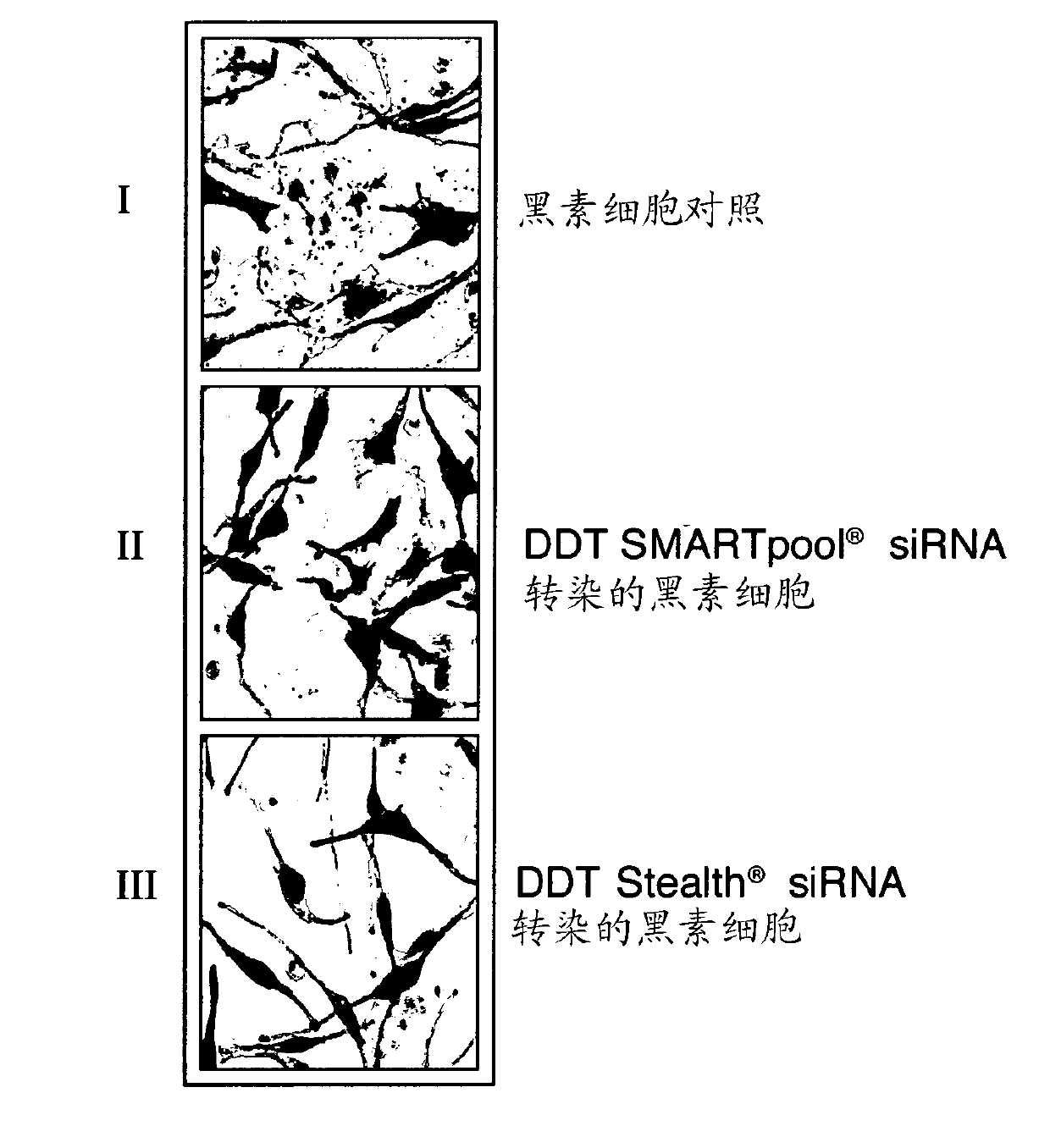
[0073] Melanosome transfer was assessed using a co-culture assay. Human epidermal melanocytes were grown to 60-80% confluence. Cells were transfected with siRNA (small interfering RNA) according to Sequitur Inc. industry-recognized and available procedures, and subsequently overlaid with HaCaT keratinocytes 72 hours later. The co-cultures were grown in phorbol ester-free keratinocyte medium (such as that available from PromoCell GmbH) and fixed 48 h after overlay to detect melanin by the Fontana-Masson staining procedure. Slides were observed using a light microscope (Carl Zeiss MicroImaging GmbH) and images were acquired using the industry-accepted AxioVision software. Ten random high power fields were analyzed per co-culture sample.
[0074] Co-culture experiments to test melanosome transfer in DDT siRNA-transfected melanocytes confirmed that melanosome transfer was reduced (inhibited) after DDT knockdown (knockdown), surprisingly showing that DDT is Plays a role in body ...
Embodiment 2
[0076] Preparation and detection of D-dopachrome substrate
[0077] In an amber 5 mL vial in H 2 Prepare a 125-mM sodium periodate working solution in O (prepared fresh on the day of the assay). Weigh 4.3 mg of dihydroxy-D-phenylalanine (D-dopa) into a 20 mL scintillation vial fitted with a pierceable septum screw cap. Using a liquid transfer cannula, first transfer 12 mL of degassed buffer into a 15 mL graduated conical tube and subsequently into a scintillation vial containing D-dopa. While stirring under nitrogen, D-dopa was dissolved for 10 minutes. The resulting working solution was 1.8 mM. Once sodium periodate is added to dissolved D-dopa, the resulting D-dopachrome is immediately used to prevent substrate degradation.
[0078] Concentrations are based on a final reaction volume of 200 microliters. Add a total of 75 µl of buffer (100 mM potassium phosphate, pH 6.8) to each well of a clear flat bottom 96-well microtiter plate. Add 25 µl of 10% glycerol / assay buffer...
Embodiment 3
[0084] Prepare a stock solution of 10 mM L-dopa (3,4-dihydroxyphenylalanine, Sigma Cat #D9628) in sodium phosphate buffer (100 mM, pH 7.0) and 0.1 mg / ml (605 units / ml ) stock solution of mushroom tyrosinase (Sigma cat #T7755) in phosphate buffered saline and stored at 4°C until use.
[0085] Test compounds (dissolved in DMSO) were first diluted in phosphate buffered saline to a working concentration of 1 mM. For each assay, 150 microliters of phosphate buffer, 10 microliters of L-dopa stock solution, and 20 microliters of each compound were added to each well of a 96-well clear bottom microtiter plate and mixed three times. Add 20 μl of mushroom tyrosinase stock solution, mix and read absorbance at 475 nm at 0, 2, 4 and 6.5 min. Plot the points as absorbance versus time and calculate the slope of the line segment. Values are expressed as a percentage of the respective untreated control response. The final DMSO concentration in each sample was less than or equal to 1.0%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com