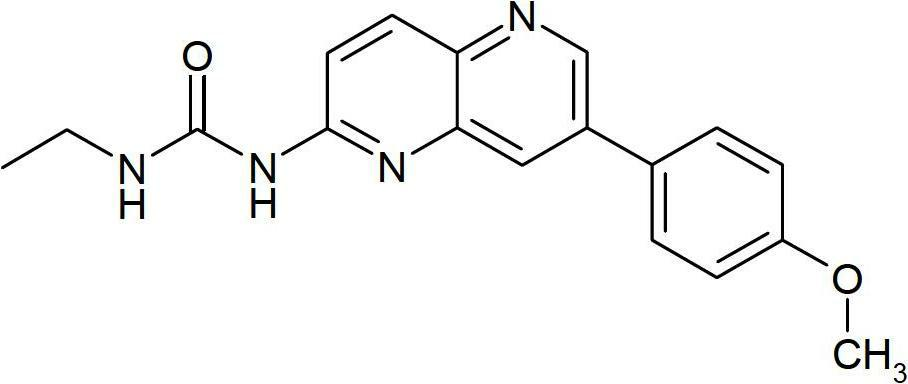
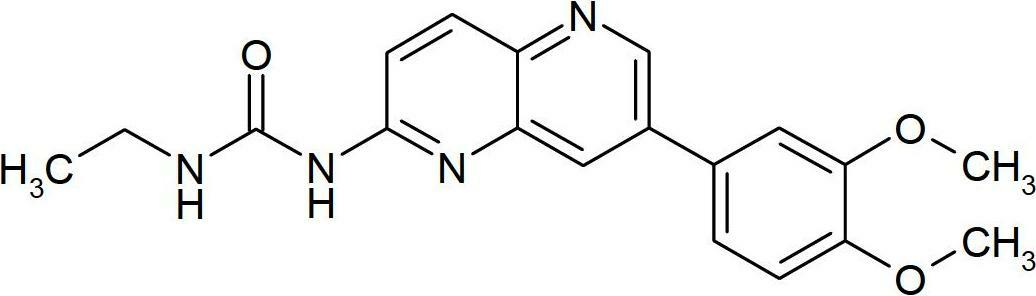
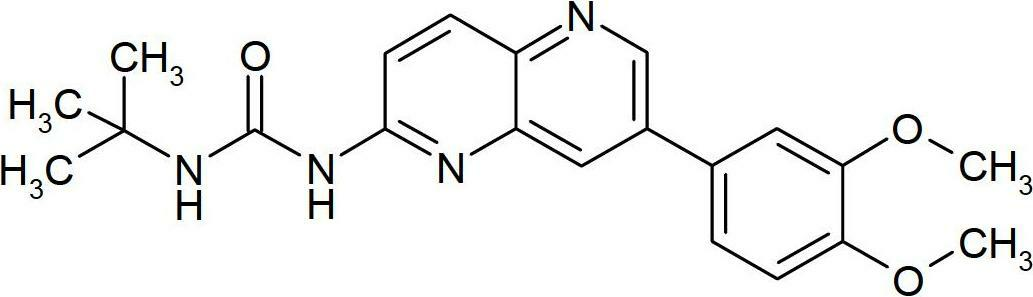
Novel naphthyridine derivatives and the use thereof as kinase inhibitors
A derivative, naphthyridine technology, applied in the field of new naphthyridine derivatives and their use as kinase inhibitors, can solve the problems of cell death, uncontrolled cell growth and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1 (reaction according to scheme 1, step 1):
Embodiment 111 and Embodiment 11
[0192] Example 1.1.1 and Example 1.1.2: 3-bromo-[1,5]naphthyridine-5-oxide and 3-bromo-1,5-naphthyridine-1-oxide
[0193]
[0194] 4.43 g (21.2 mmol, 1 equivalent) of 3-bromo-1,5-naphthyridine (W. Czuba, Recueil des Travaux Chimiques des Pays-Bas 1963, 82, 988-996) were introduced into 165 mL of dichloromethane. Then 5.23 g (21.2 mmol, 1 equiv) of m-chloroperbenzoic acid were added portionwise at 0°C. The mixture was stirred at room temperature for 18 h. The mixture was washed with 1M aqueous NaOH and water. The organic layer was washed with Na 2 SO 4 Dry, filter and evaporate to dryness. The residue was purified by column chromatography using dichloromethane and then dichloromethane / ethanol:98 / 2 as eluents. The solvent was evaporated to dryness to give 3.08 g of 3-bromo-1,5-naphthyridine-5-oxide (pale yellow powder, 64% yield) and 1.00 g of 3-bromo-1,5-naphthyridine- 1-oxide (yellow powder, 21% yield).
[0195] 3-Bromo-[1,5]naphthyridine-5-oxide
[0196] Yield: 3.0...
Embodiment 2
[0205] Example 2 (reaction according to scheme 1, step 2):
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com