Polypeptide medicine against hepatitis B virus X protein
A hepatitis B virus, anti-hepatitis B technology, applied in the direction of viral peptides, antiviral agents, viruses, etc., can solve the problems of short half-life of polypeptide fragments, affecting pharmacodynamics, and difficulty in discovering polypeptide drugs, and achieve obvious efficacy. effect of learning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Design and preparation of polypeptides
[0101] Synthetic polypeptide functional fragment anti-HBxP2#:
[0102] The present invention has synthesized the polypeptide (hereinafter referred to as Anti -HBxP2#). The preparation of the polypeptide adopts a solid-phase synthesis method, such as using an AAPPTECAPex396 polypeptide synthesis instrument (purchased from Hong Kong Universal Analytical and Testing Instrument Co., Ltd.), in a closed explosion-proof glass reactor to make amino acids according to the sequence shown in SEQ ID NO: 1, From C-terminus-carboxyl-terminus to N-terminus-amino-terminus, this refers to the first one added to the amino acid sequence Pro-Asp-Leu-His-Lys-Asn-Glu-Leu-Lys-His-Val-Lys-Tyr The amino acid monomer is Tyr at the C-terminus, then Lys, then Val, until the last Asp and Pro at the N-terminus, which are continuously added, reacted, synthesized, and operated to finally obtain the desired amino acid sequence. The solid-phase synt...
Embodiment 2
[0109] Embodiment two: the anti-HBx activity of isolated (in vitro) polypeptide
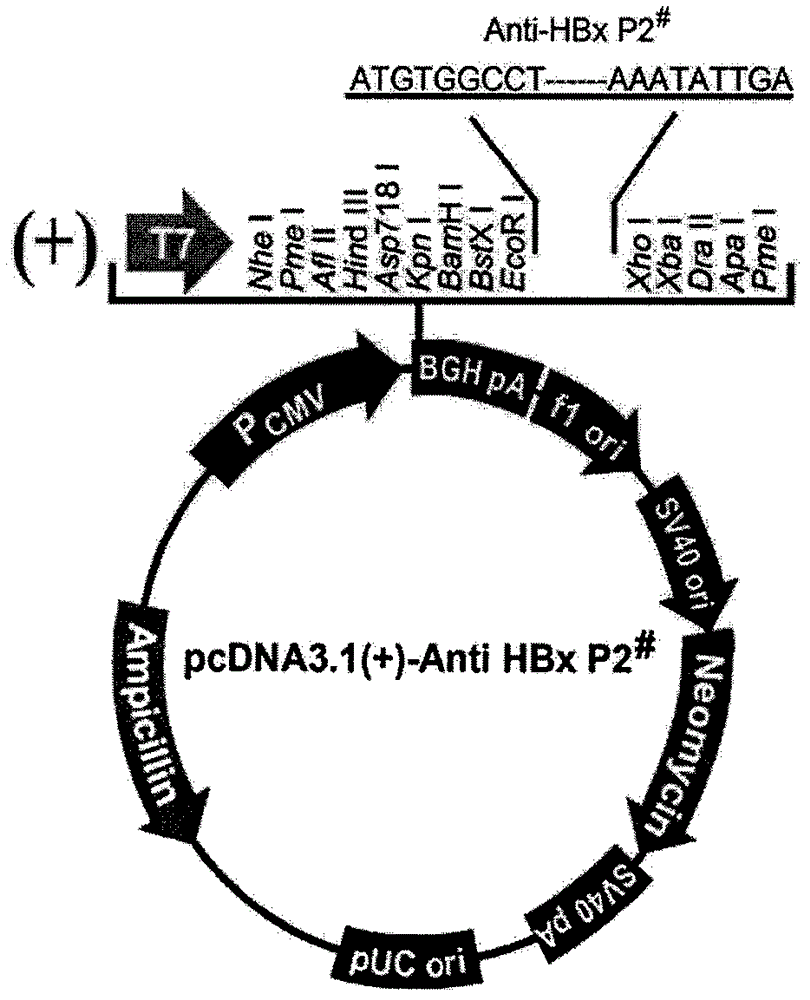
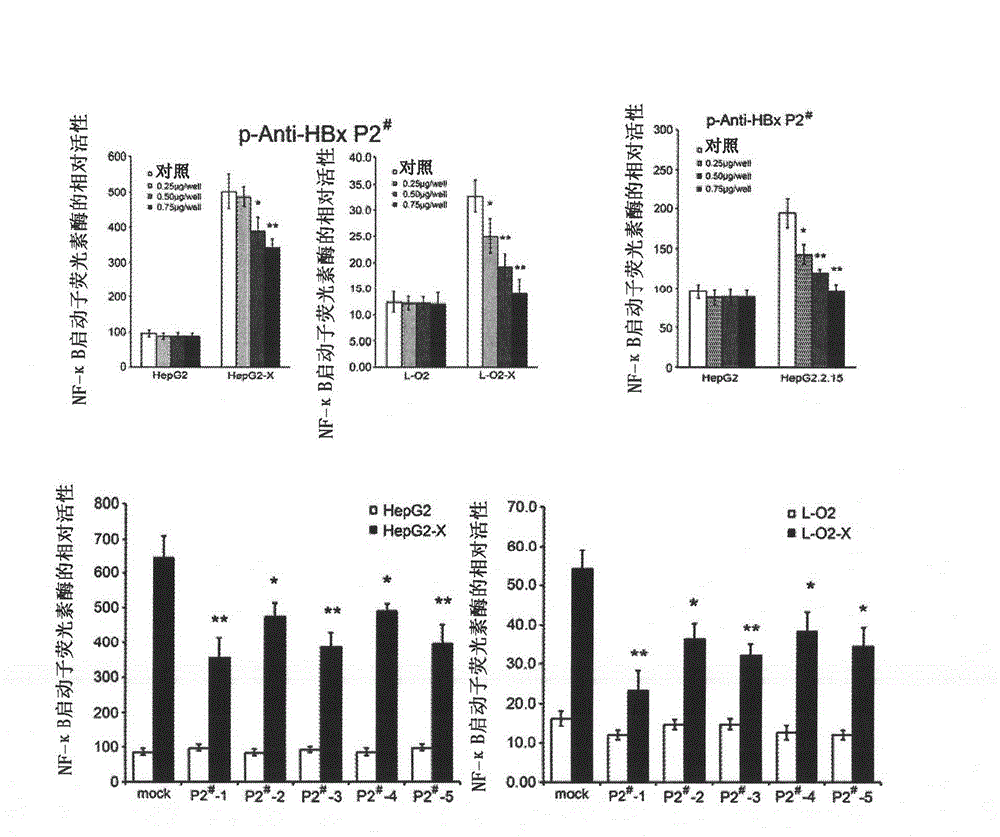
[0110] Two methods are used to detect the anti-HBx activity of the polypeptide in Example 1 in vitro: the first method is to use molecular cloning technology to clone the cDNA expressing the polypeptide in Example 1 into the eukaryotic expression vector pcDNA3.1 On (+), through gene transfection, the purpose of expressing the studied polypeptide in liver cancer cells is realized, and then the effect of the researched polypeptide on inhibiting HBx is observed; the second method is to use artificially synthesized polypeptides and directly add them to cultured liver cancer cells In the culture medium, observe the effect of polypeptide on inhibiting HBx.
[0111] There are two kinds of liver cancer cells used in the experiment: one is liver cancer HepG2-X cells constitutively expressing HBx (hepatoma HepG2 cells stably transfected with HBx); the other is liver cancer HepG2.2.15 cells constitutively e...
Embodiment 3
[0190] Example 3: Experiments on the effectiveness of polypeptides in vivo
[0191] HepG2-X cells or HepG2.2.15 cells in the logarithmic growth phase were digested with trypsin to make a cell suspension, the number of cells was calculated, and the cells were diluted to 1×10 with sterile normal saline. 7 cells / ml and stored in ice water. Twelve 4- to 6-week-old female BALB / C nude mice were then randomly divided into 2 groups: ① Control group, subcutaneously inject 0.2ml of the above-mentioned diluted cells in the armpit of the right forelimb of each mouse, and only inject 0.5 ml sterilized distilled water (without polypeptide drugs); ② experimental group (administration dose is 10 mg / kg body weight). Subcutaneously inject 0.2ml of the above-mentioned diluted cells into the armpit of the right forelimb of each mouse, and after 7 days of injection, the tumor volume (V=L×W 2 ×0.5) up to 100mm 3 , and then subcutaneously inject the above polypeptide drugs (dissolve the freeze-dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com