A polypeptide that antagonizes the binding protein of hepatitis B virus X protein, a drug comprising the polypeptide and its application
A hepatitis B virus, protein-binding technology, applied in drug combinations, anti-tumor drugs, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Design of activatable tumor targeting cell penetrating peptide (activatablecellpenetratingpeptide, ACPP)
[0060] Cell penetrating peptides (CPPs) are currently the strongest and fastest transmembrane transport molecules. They have the characteristics of water solubility, low lysis, and enter various cell membranes through non-phagocytosis. They bring new technologies to the treatment of tumors. opportunity. Hairpin-shaped composite CPPs modified on the basis of CPPs are called tumor-targeting CPPs, also known as activatable cellpenetrating peptides (ACPPs).
[0061] The sequence of the ACPP adopted in the present invention is:
[0062] SEQ ID No.7: Val-Ser-Arg-Arg-Arg-Arg-Arg-Arg-Ser-Ser-Arg-Arg-Arg-Arg-Pro-Leu-Gly-Leu-Ala-Gly-Asp-Asp-Asp- Asp-Gly-Gly-Glu-Glu-Glu-Glu-Glu-Glu.
[0063] Structural features: (1) active center CPP region (ValSerArgArgArgArgArgArgArgSerSerArgArgArgArgArg) with cell membrane penetration function (positively charged sequence); (2...
Embodiment 2
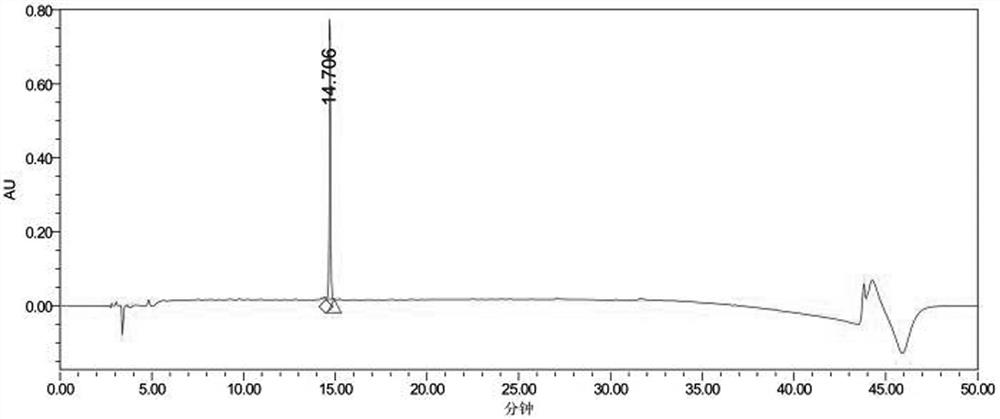
[0065] Example 2 Solid phase synthesis and purification of breast cancer treatment polypeptide drug ACPP-RI-HBXIP
[0066] ACPP-RI-HBXIP polypeptide sequence (SEQIDNo.8): Val-Ser-Arg-Arg-Arg-Arg-Arg-Arg-Ser-Ser-Arg-Arg-Arg-Arg-Pro-Leu-Gly-Leu-Ala -Gly-Asp-Asp-Asp-Asp-Gly-Gly-Glu-Glu-Glu-Glu-Glu-Glu-Gly-Pro-Lys-Leu-Ser-Arg-Gln-Glu-Ser-Ala-Glu-Thr -His-Lys-Gly-Asn-Arg-Met-Tyr.
[0067] (1) Materials and reagents
[0068] Fmoc-Tyr(tBu)-Wang resin, substitution value 0.44mmol / g.
[0069] Required protected amino acids: Fmoc-Val-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Met-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gly- OH, Fmoc-Lys(Boc)-OH, Fmoc-His(Trt)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Ser(tBu) -OH, Fmoc-Gln(Trt)-OH, Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Val-OH.
[0070] Reagents: HOBt, DIC, DMF, piperidine.
[0071] (2) Instruments
[0072] PSI300 peptide synthesizer, Waters high performance liquid chromatography, magnetic stirrer.
[0073] (3) ...
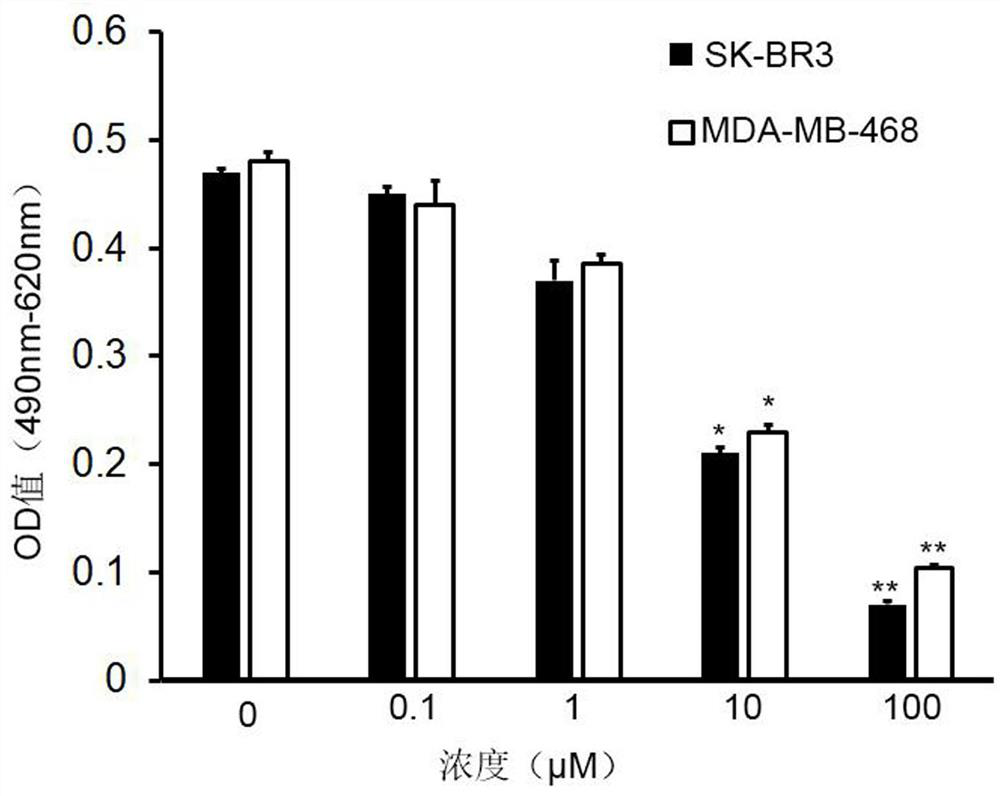
Embodiment 3
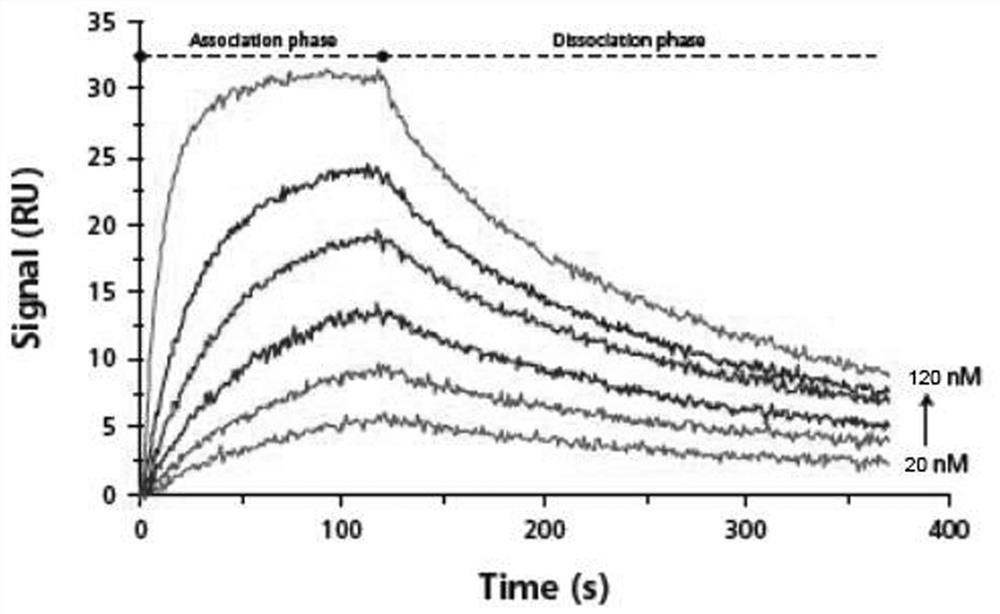
[0088]Using surface plasmon resonance technology, the biosensor Biacore T200 biomacromolecular interaction analyzer (manufactured by GE Healthcare) was used to determine the binding force between the synthetic polypeptide ACPP-RI-HBXIP and the recombinantly expressed Recombinant HumanHBXIP protein (Abcam, No. ab130038) in vitro. Determination. Dilute the Recombinant Human HBXIP protein with NaAc with an optimal pH of 5.0, select the optimal dilution and select a channel on the CM5 chip for coupling, the target value of the coupled Recombinant Human HBXIP is 1000RU, and select another channel as a control. The test conditions were 25°C, the flow rate was 20 μL / min, and the buffer was HBS-EP (pH7.4, Bia-Certified). After the chip-coupled RecombinantHuman HBXIP protein reaches the target value, the surface of the chip is blocked, and then the ACPP-RI-HBXIP polypeptide protein is diluted with HBS-EP buffer, and protein samples with different concentrations (6 different concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com