Method for preparing lead chloride and zinc sulfate by using mid low grade zinc oxide ores and zinc oxide-lead oxide paragenetic ores
A technology of zinc oxide ore and lead chloride, which is applied in the direction of zinc sulfate and lead halide, can solve the problems of ammonia distillation tower scarring, single product structure, and high energy consumption in the ammonia distillation process, and achieve low production costs and low equipment requirements. High, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
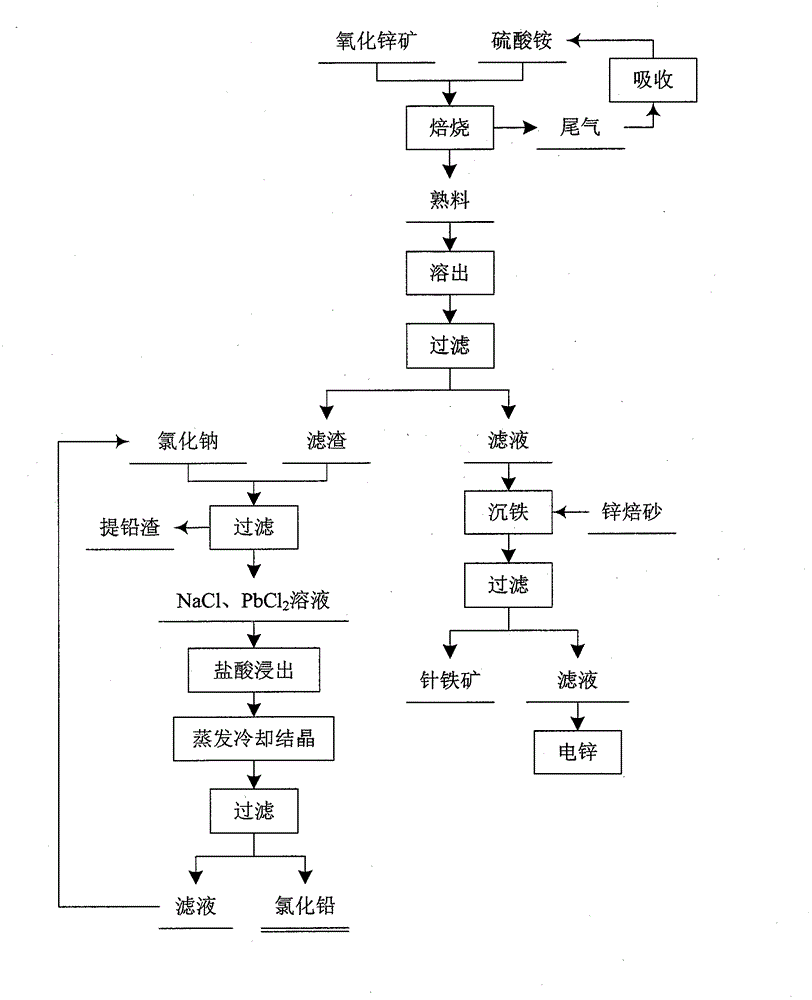
[0029] The zinc ore composition used is: ZnO 30.6%, SiO 2 21.70%, Fe 2 o 3 20.10%, Al 2 o 3 4.70%, PbO 4.67%, CaO 4.26%, SrO 3.56%.
[0030] Grind zinc ore to below 80μm and mix evenly with ammonium sulfate. The amount of ammonium sulfate added is 0.8 times the theoretical value of ammonium sulfate required for the complete reaction of zinc, iron and aluminum in the zinc oxide ore, and the roasting temperature is 350°C for 3 hours. The generated tail gas is absorbed by sulfuric acid and then returned to the roasting process.
[0031] The clinker is eluted with water, the liquid-solid mass ratio is 2:1, the elution time is 2 hours, the temperature is 100° C., and it is filtered after the elution is completed. Add hydrogen peroxide to oxidize Fe in the obtained filtrate 2+ , and spray it into the bottom solution at a certain flow rate to keep the Fe in the bottom solution 3+ The concentration is below 1g / L, the pH of the solution is adjusted to 3.5 with zinc calcine, ...
Embodiment 2
[0034] The zinc ore composition used is: ZnO 35.82%, SiO 2 18.05%, Fe 2 o 3 14.06%, Al 2 o 3 5.10%, PbO 6.84%, CaO 3.67%, SrO 2.24%.
[0035] Grind zinc ore to below 80μm and mix evenly with ammonium sulfate. The amount of ammonium sulfate added is 1.1 times the theoretical value of ammonium sulfate required for the complete reaction of zinc, iron and aluminum in the zinc oxide ore. The roasting temperature is 400°C and the time is 2.5 hours. The generated tail gas is absorbed by sulfuric acid and then returned to the roasting process.
[0036] The clinker is eluted with water, the liquid-solid mass ratio is 4:1, stirring is carried out during the elution process, the elution time is 1.5 h, the temperature is 80° C., and filtering is performed after the elution is completed. Add hydrogen peroxide to oxidize Fe in the obtained filtrate 2+ , and spray it into the bottom solution at a certain flow rate to keep the Fe in the bottom solution 3+ When the concentration is ...
Embodiment 3
[0039] The zinc ore composition used is: ZnO 25.5%, PbO 10.34%, SiO 2 20.41%, Fe 2 o 3 16.90%, Al 2 o 3 6.99%, CaO 5.80%, SrO 2.98%.
[0040] Grind zinc ore to below 80μm and mix evenly with ammonium sulfate. The amount of ammonium sulfate added is 1.5 times the theoretical value of ammonium sulfate required for the complete reaction of zinc, iron and aluminum in the zinc oxide ore, and the roasting temperature is 500°C for 1 hour. The tail gas produced by roasting is absorbed by sulfuric acid, and then returned to the roasting process. The clinker is eluted with water, the liquid-solid mass ratio is 5:1, stirring is carried out during the elution process, the elution time is 0.5h, the temperature is 20°C, and the elution is completed and filtered.
[0041] Add hydrogen peroxide to oxidize Fe in the obtained filtrate 2+ , and spray it into the bottom solution at a certain flow rate to keep the Fe in the bottom solution 3+ When the concentration is below 1g / L, use zi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com