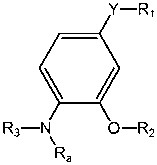
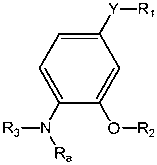
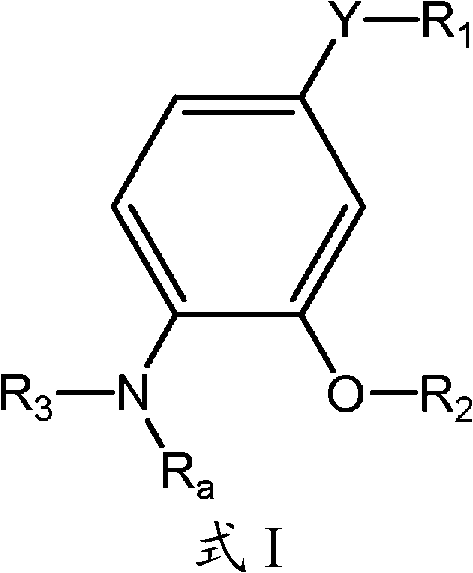
4-substituted-2-phenoxy-phenylamine delta opioid receptor modulators
A Substitute, Phenyl Technology, Applied in the Field of Novel Opioid Receptor Modulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0373]
[0374] A. 4-Bromo-2-(4-methoxy-phenoxy)nitrobenzene (1c). Compound 1a (2.20g, 10.0mmol), 4-methoxyphenol (compound 1b, 1.32g, 10.5mmol), K 2 CO 3 (1.52 g, 11.0 mmol) and 6 mL of DMF were stirred at 75°C for 3 hours. The mixture was concentrated in vacuo and the residue was partitioned between EtOAc and water. The organic layer was sequentially washed with 2N NaOH aqueous solution, 2N HCl aqueous solution, saturated NaHCO 3 aqueous and brine washes, with Na 2 SO 4 Dried, then concentrated to give compound 1c (2.95 g, 91%) as a brown gel. 1 H NMR (300MHz, CDCl 3 ): δ7.11-7.84 (d, 1H), 7.24-7.27 (m, 1H), 7.01-7.06 (m, 3H), 6.93-6.97 (m, 2H), 3.71 (s, 3H).
[0375] B. 4-Bromo-2-(4-methoxy-phenoxy)-aniline (1d). A mixture of compound 1c (1.64 g, 5.06 mmol), zinc (1.98 g, 30.4 mmol), 15 mL of HOAc and 50 mL of MeOH was stirred at 20 °C for 20 hours. After removal of solvent, the residue was partitioned between EtOAc and 3N aqueous NaOH. The organic phase was wa...
example 2
[0382]
[0383] A. 2-(S)-{[4-(2-Diethylcarbamoyl-ethyl)-2-(4-methoxy-phenoxy)-anilino]-methyl}-pyrrolidine - tert-butyl 1-carboxylate (2a). A mixture of compound Ik (0.050 g, 1.0 mmol) and 10% palladium on carbon in MeOH was shaken at 20 °C for 4 hours under a hydrogen atmosphere (34 psi). The catalyst was filtered off and the solvent was removed by evaporation to give compound 2a. MS: m / z526.3 (M+H) + .
[0384] B. Compound 2: N,N-diethyl-3-[3-(4-methoxyphenoxy)-4-{[(2S)-pyrrolidin-2-ylmethyl]amino}phenyl ] Propionamide. Compound 2a (0.050g, 0.095mmol), TFA and CH 2 Cl 2 The mixture was stirred at 20 °C for 4 hours. After concentration, the residue was purified by reverse phase HPLC to give compound 2 (0.022 g, 36% yield) as TFA salt. 1 H NMR (300MHz, CD 3 OD): δ6.87-6.91(m, 5H), 6.78(d, 1H), 6.60(d, 1H), 3.90(m, 1H), 3.79(s, 3H), 3.42-3.48(m, 2H) 3.24-3.34(m, 6H), 2.77(t, 2H), 2.53(t, 2H), 2.18-2.28(m, 1H), 2.00-2.14(m, 2H), 1.73-1.86(m, 1H), 1.03-1.08 (m, 6H);...
example 3
[0386]
[0387] A. 4-Chloro-2-(4-methoxy-phenoxy)nitrobenzene (3b). 4-chloro-2-fluoronitrobenzene (compound 3a, 1.76g, 10mmol), compound 1b (1.30g, 10.5mmol) and K 2 CO 3 (1.52 g, 11 mmol) was heated in 6 mL of DMF at 75 °C for 3 hours. The mixture was concentrated in vacuo and the residue was partitioned between EtOAc and water. The organic layer was sequentially washed with 1N NaOH aqueous solution, 1N HCl aqueous solution, saturated NaHCO 3 aqueous and brine washes, followed by NaXSO 4 dry. Flash column chromatography (SiO 2 ) was concentrated and purified to obtain compound 3b (2.75 g, 98% yield) as a yellow solid. MS: m / z 279.9 (M+H) + .
[0388] B. 4-Chloro-2-(4-methoxy-phenoxy)-aniline (3c). A mixture of compound 3b (2.47 g, 8.83 mmol), zinc (3.46 g, 53 mmol), 60 mL HOAc, 5 mL THF and 36 mL MeOH was stirred at 20° C. for 20 hours. The solid material was filtered and washed with MeOH. The filtrate was partitioned between EtOAc and 1N aqueous NaOH. The orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com