Novel synthetic method of key intermediate2-anisacetone of loxoprofen
A technology of tolylpropionic acid and synthesis method, applied in the direction of organic chemistry, nitrile preparation, etc., can solve the problems of unstable quality, poor purity, affecting the purity and efficiency of loxoprofen, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
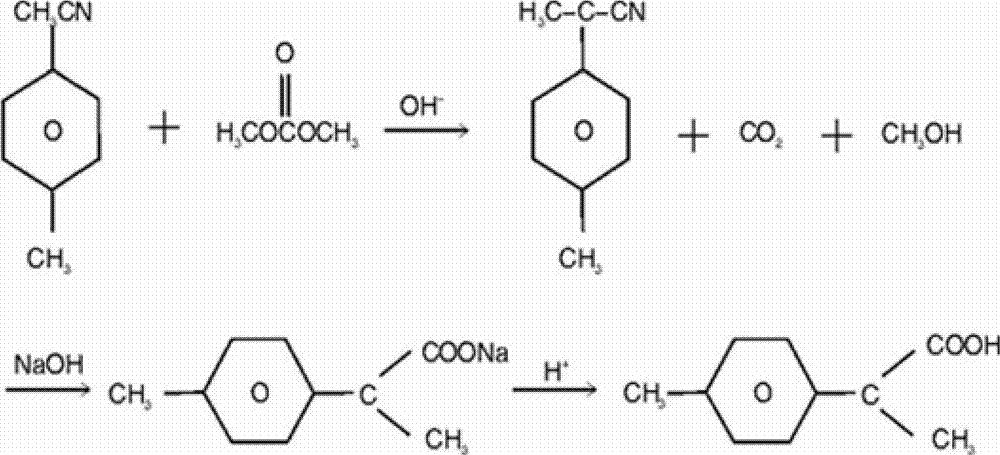
[0071] Synthesize 2-p-tolylpropionic acid as follows:
[0072] Put 104kg of p-tolueneacetonitrile, 540kg of dimethyl carbonate, and 280kg of potassium hydroxide into a stainless steel reactor for α-methylation reaction; raise the temperature to 175°C, and control the pressure to 18kg / cm 2 , insulation 8h, generate α-methyl-p-toluene acetonitrile; be cooled to normal temperature, filter out potassium carbonate solid, reclaim dimethyl carbonate, obtain the mother liquor that leaves α-methyl-p-toluene acetonitrile; Add 400kg concentration to be 15wt% sodium hydroxide aqueous solution, heated to 110°C for reflux for 8 hours; cooled to 60°C, added 200kg of toluene to extract and remove organic matter, moved the water layer into a clean reaction kettle; added dropwise 15wt% hydrochloric acid to adjust the pH value to 1-2; cool to 5°C, stir and filter, and the obtained off-white solid crystal is 2-p-tolylpropionic acid.
[0073] It was determined that 158kg of 2-p-tolylpropionic aci...
Embodiment 2
[0075] Synthesize 2-p-tolylpropionic acid as follows:
[0076] Put 100kg of p-tolueneacetonitrile, 500kg of dimethyl carbonate, and 250kg of potassium hydroxide into a stainless steel reactor for α-methylation reaction; raise the temperature to 180°C, and control the pressure to 16kg / cm 2 , keep warm for 8.5h to generate α-methyl-p-tolueneacetonitrile; cool to normal temperature, filter out potassium carbonate solid, recycle dimethyl carbonate, and obtain the mother liquor with α-methyl-p-tolueneacetonitrile; add 440kg concentration 16wt% sodium hydroxide aqueous solution, heated to 108°C for 7h under reflux for hydrolysis; cooled to 58°C, added 240kg of toluene to extract and remove organic matter, moved the water layer into a clean reaction kettle; added dropwise 20wt% hydrochloric acid to adjust the pH value to 1-2; cooled to 6 ° C, stirred, filtered, the obtained off-white solid crystal is 2-p-tolylpropionic acid.
[0077] It was determined that 156kg of 2-p-tolylpropioni...
Embodiment 3
[0079] Synthesize 2-p-tolylpropionic acid as follows:
[0080] Put 100kg of p-tolueneacetonitrile, 450kg of dimethyl carbonate, and 200kg of potassium hydroxide into a stainless steel reactor for α-methylation reaction; raise the temperature to 165°C, and control the pressure to 15kg / cm 2 , insulation 7h, generate α-methyl-p-tolueneacetonitrile; be cooled to normal temperature, filter out potassium carbonate solid, reclaim dimethyl carbonate, obtain the mother liquor that leaves α-methyl-p-tolueneacetonitrile; Add 350kg concentration to be 18wt% sodium hydroxide aqueous solution, heated to 105°C for reflux hydrolysis for 6 hours; cooled to 55°C, added 150kg of toluene to extract and remove organic matter, moved the water layer into a clean reaction kettle; added dropwise 10wt% hydrochloric acid to adjust the pH value to 1-2; cool to 2°C, stir and filter, and the obtained off-white solid crystal is 2-p-tolylpropionic acid.
[0081] It was determined that 155kg of 2-p-tolylprop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com