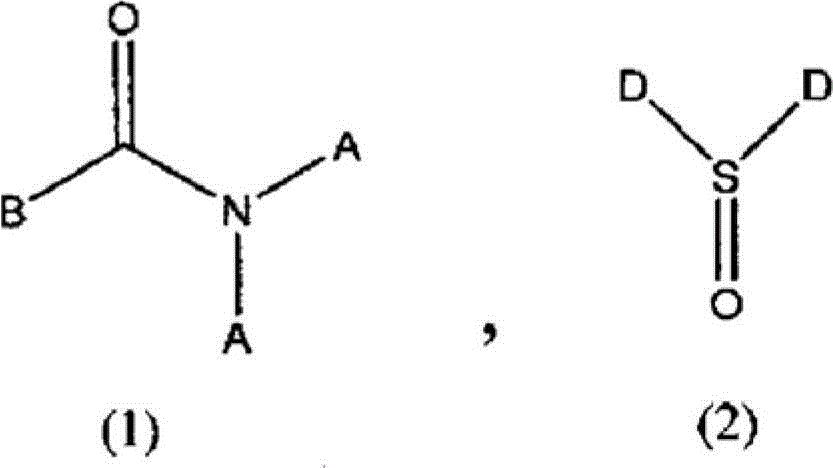
Method for producing difluoroacetic acid ester
A technology of difluoroacetic acid ester and manufacturing method, which is applied to the preparation of carboxylic acid ester, the preparation of carboxylic acid halide, chemical instruments and methods, etc., can solve the problems of reducing the recovery rate, increasing the solubility, etc. Comprehensive yield, the effect of avoiding yield reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
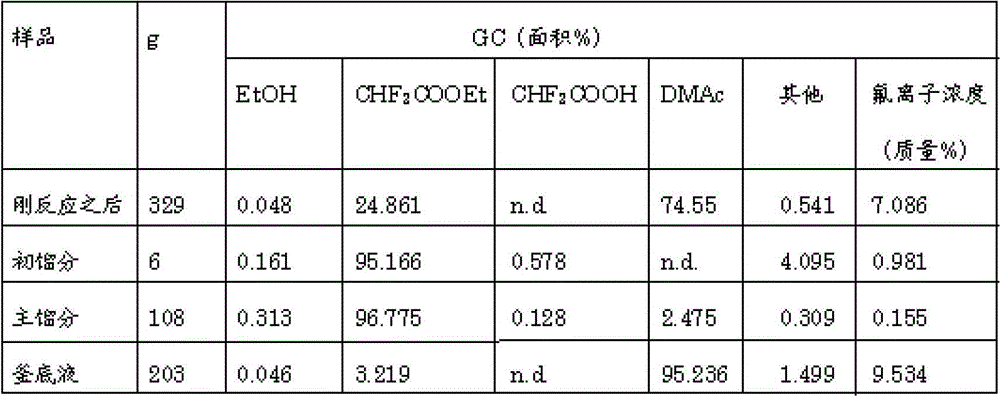
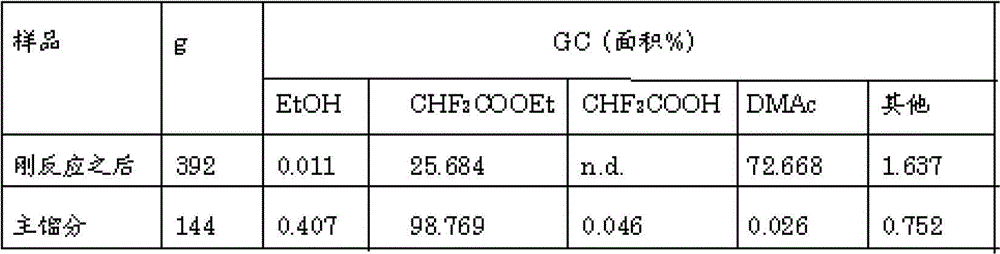
[0069] The present invention will be described below using examples, but the embodiments of the present invention are not limited thereto. In the examples, ethyl difluoroacetate (CHF 2 COOEt), ethanol (EtOH), difluoroacetic acid (CHF 2 COOH) and other organic components are measured by FID gas chromatography (GC), and the content of inorganic acids such as hydrogen fluoride is measured by ion chromatography (IC).
Synthetic example 1
[0071] Aluminum phosphate (Aluminum phosphate) produced by Aldrich was pelletized into pellets with a diameter of 5 mm x a length of 5 mm, and fired at 700° C. for 5 hours in a nitrogen stream to prepare an aluminum phosphate catalyst. 2200 cc of this was filled in a large gas phase reaction tube (made of stainless steel, inner diameter 43 mm x length 1800 mm) equipped with a vaporizer. While flowing nitrogen gas at a rate of 1000 cc / min, the reaction tube was heated by an external electric furnace. After the temperature of the catalyst reached 50° C., hydrogen fluoride (HF) was introduced at a maximum rate of 6 g / min through the vaporizer while monitoring so as not to generate sudden heat generation. In the state of circulating HF, slowly raise the temperature to 300°C, slowly increase the HF supply rate to 12g / min, and keep it at 300°C for 72 hours, after that, lower the set temperature of the heater, and the internal temperature reaches 250°C , stop the flow of HF, increas...
Synthetic example 2
[0073] Aluminum phosphate (Aluminum phosphate) produced by Aldrich was pelletized into pellets with a diameter of 5 mm x a length of 5 mm, and fired at 700° C. for 5 hours in a nitrogen stream to prepare an aluminum phosphate catalyst. 200 cc of this was filled in a small gas phase reaction tube (made of stainless steel, inner diameter 37 mm x length 500 mm) equipped with a vaporizer. While flowing nitrogen gas at a rate of 100 cc / min, the reaction tube was heated by an external electric furnace. After the temperature of the catalyst reached 50° C., hydrogen fluoride (HF) was introduced through the vaporizer at a maximum rate of 0.6 g / min while monitoring so as not to generate sudden heat generation. In the state of circulating HF, slowly raise the temperature to 300°C, slowly increase the HF supply rate to 1.2g / min, and keep it at 300°C for 72 hours, after that, lower the set temperature of the heater, and the internal temperature reaches 250 At ℃, stop the flow of HF, incre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com