Cancer antigen helper peptide
An antigen and auxiliary technology, applied in the direction of cancer antigen components, tumor-specific antigens, vertebrate antigen components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
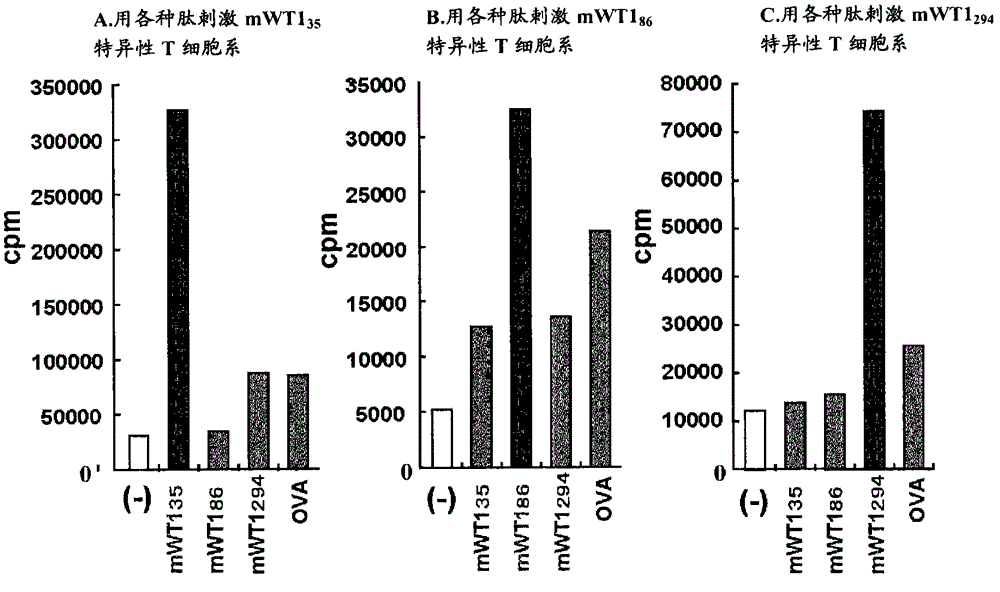
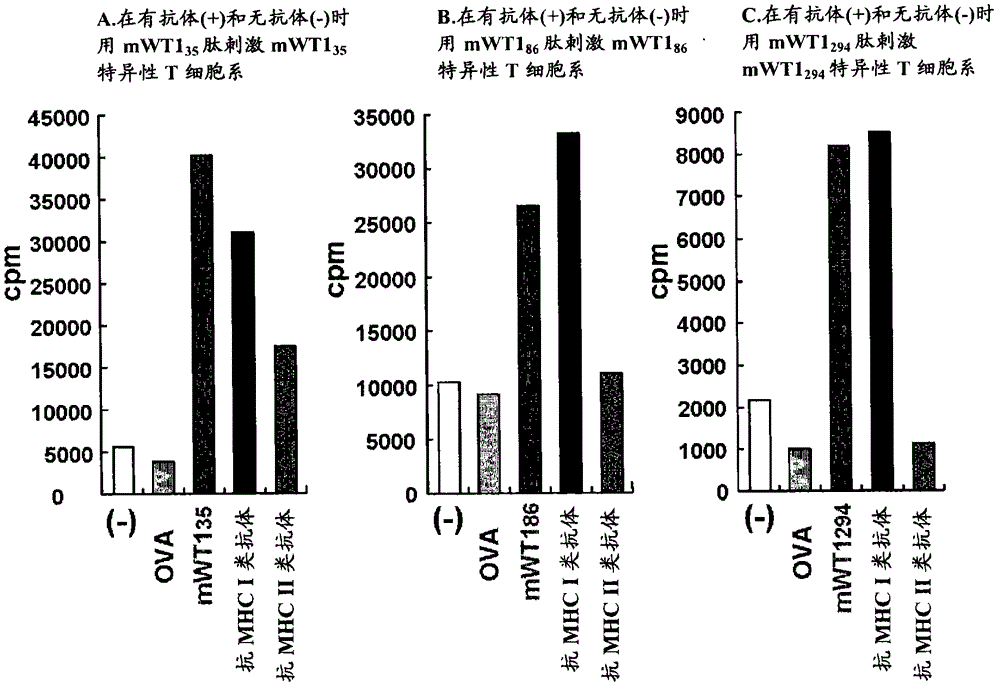
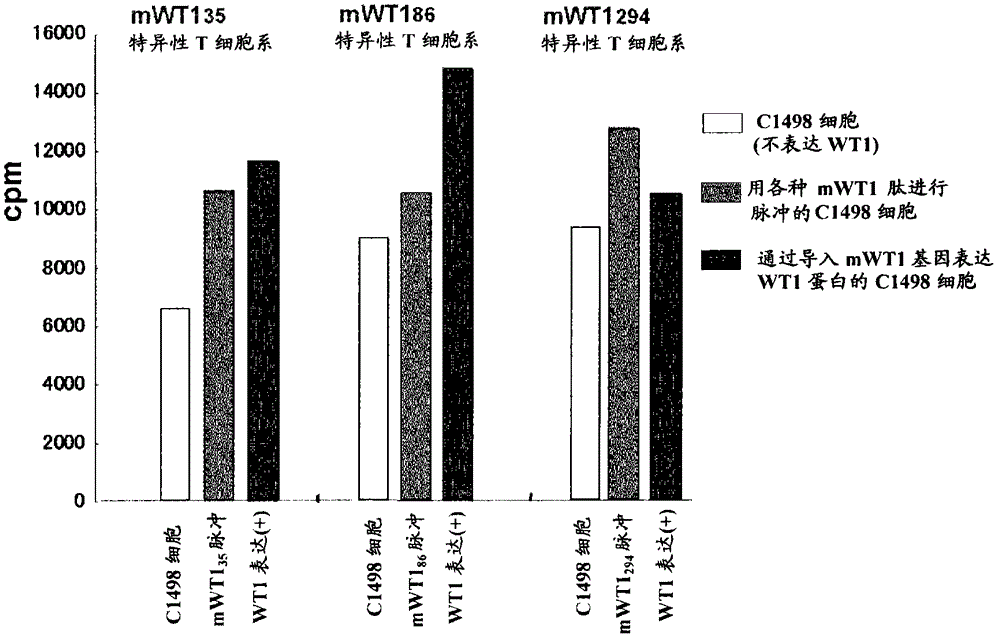
[0134] Selection of candidate WT1 peptides that bind to MHC class II molecules
[0135] To search for peptide sequences that bind to MHC class II molecules, the method shown by Rammensee et al. (Rammensee et al., Immunogenetics 41:178-228, 1995) was used. Specifically, the selection was made using the program described in the right-hand column of the table and the rule of Rammensee et al. By this method, the WT1 35 Peptides were restricted to the peptide sequences shown in Tables 1 and 2, the WT1 86 Peptides were restricted to the peptide sequences shown in Tables 3 and 4, and the WT1 294 Peptides were restricted to the peptide sequences shown in Table 5 and Table 6. The left-hand column in Tables 1-6 indicates "suitability" as a candidate peptide sequence. The larger the number of "○", the higher the suitability for the rule of Rammensee et al. No mark indicates poor fit. Similarly, the amino acid groups in brackets in the column of "candidate peptide sequences bindin...
Embodiment 2
[0161] Determination of WT1-specific cytotoxic T cells (CTL)
[0162] Mice were treated with only WT1 126 Peptide (MHC class I), WT1 126 Peptide (MHC class I) + WT1 35 Peptide (MHC class II), WT1 126 Peptide (MHC class I) + WT1 86 Peptide (MHC class II) or WT1 126 Peptide (MHC class I) + WT1 294 Peptide (MHC class II) was immunized three times, and spleen cells of mice were prepared. Then, splenocytes were treated with WT1 126 Peptide (MHC class I) stimulated once in vitro, on the 6th day, use WT1 126 RMAS cells pulsed with peptide (MHC class I) were used as target cells, and cytotoxic activity was measured. Unused WT1 126 Peptide (MHC class I) pulsed RMAS cells were used as control target cells. Therefore, with only WT1 126 Peptide (MHCI class I) immunized mouse splenocytes compared with WT1 126 The splenocytes of mice immunized with peptide (MHC class I) + WT1 helper peptide (MHC class II) more strongly induced WT1-specific cytotoxic T cells ( Figure 5 ). Th...
Embodiment 3
[0164] tumor implantation experiments
[0165] The C1498 leukemia cells expressing WT1 were divided into 2.5×10 5 The ratio of cells / mouse was subcutaneously implanted into mice, and 50 μg / mouse of WT1 was injected once a week for 3 times from one week after implantation 35 The helper peptide was administered intradermally with Freund's incomplete adjuvant ( Image 6 ). As a control, WT1 was replaced with saline 35 The helper peptide was administered intradermally with Freund's incomplete adjuvant. The size of subcutaneous tumors was measured over time, and disease-free survival was calculated until day 29 after subcutaneous implantation. As a result, tumors enlarged in all mice in the control group, whereas in WT1 35 In the helper peptide (MHC class II) immunization group, tumor proliferation was completely inhibited in 4 out of 10 mice ( Figure 7 ). Similarly, WT1 35 A significant difference was seen between the auxiliary peptide immunization group and the control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com