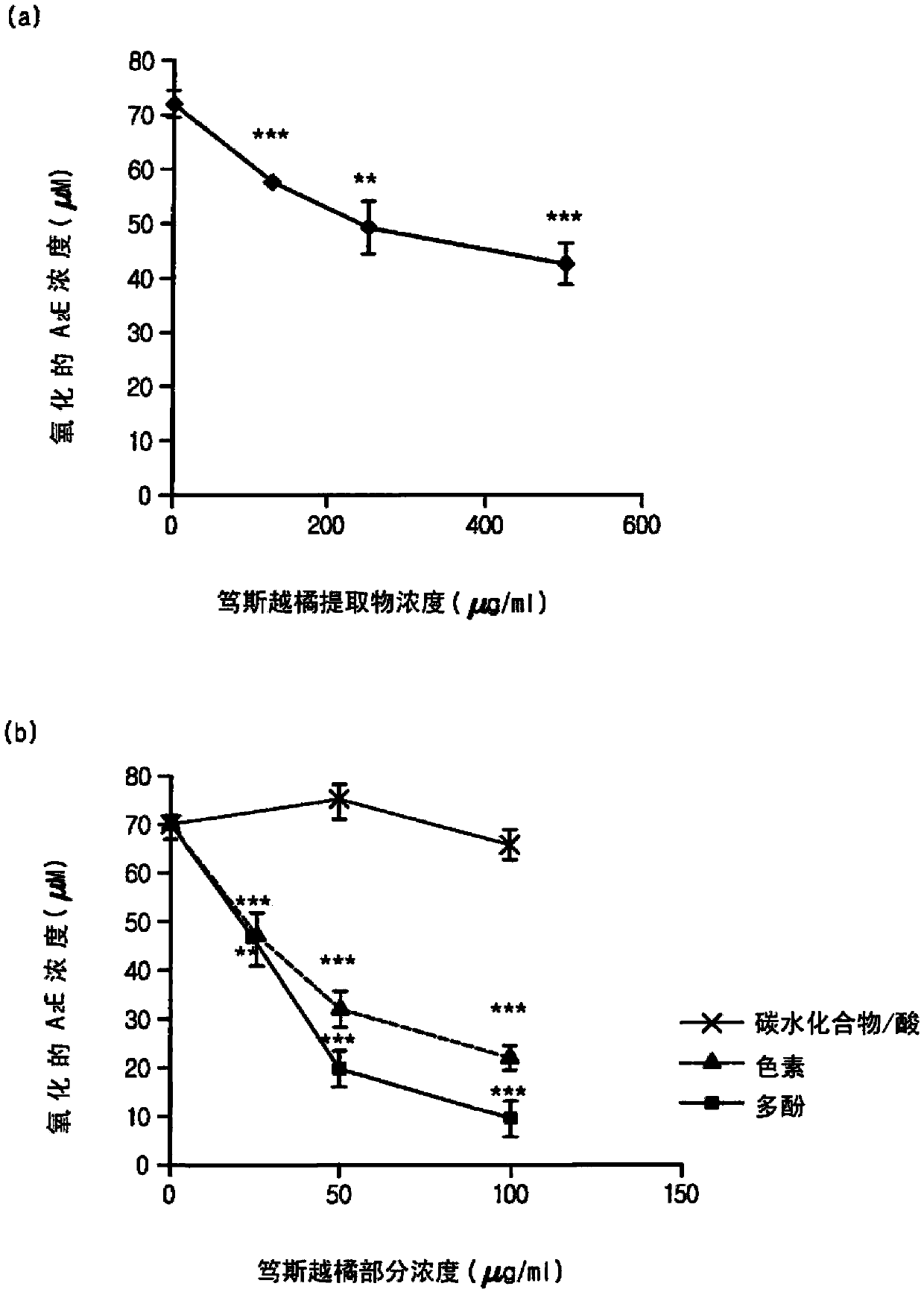
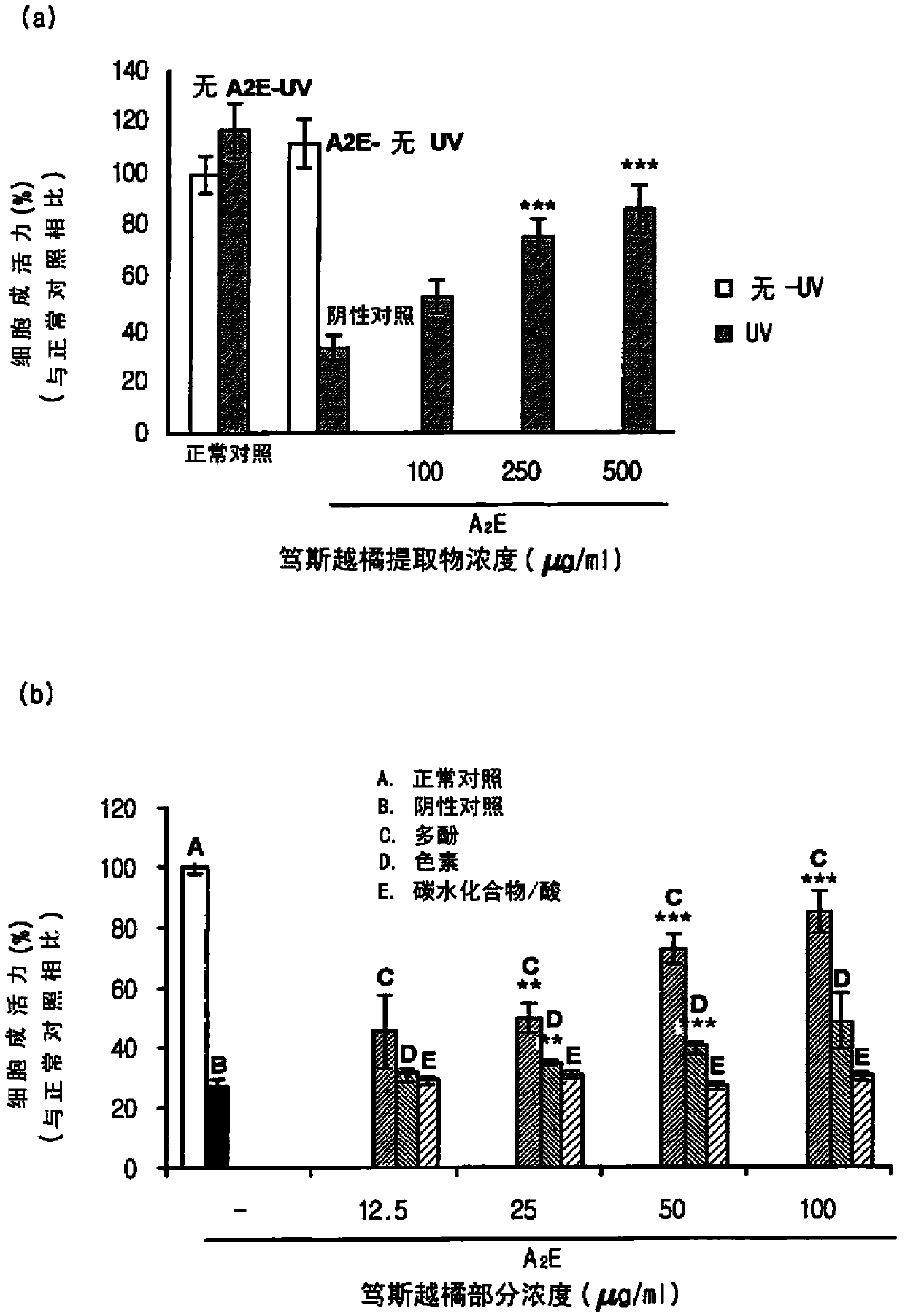
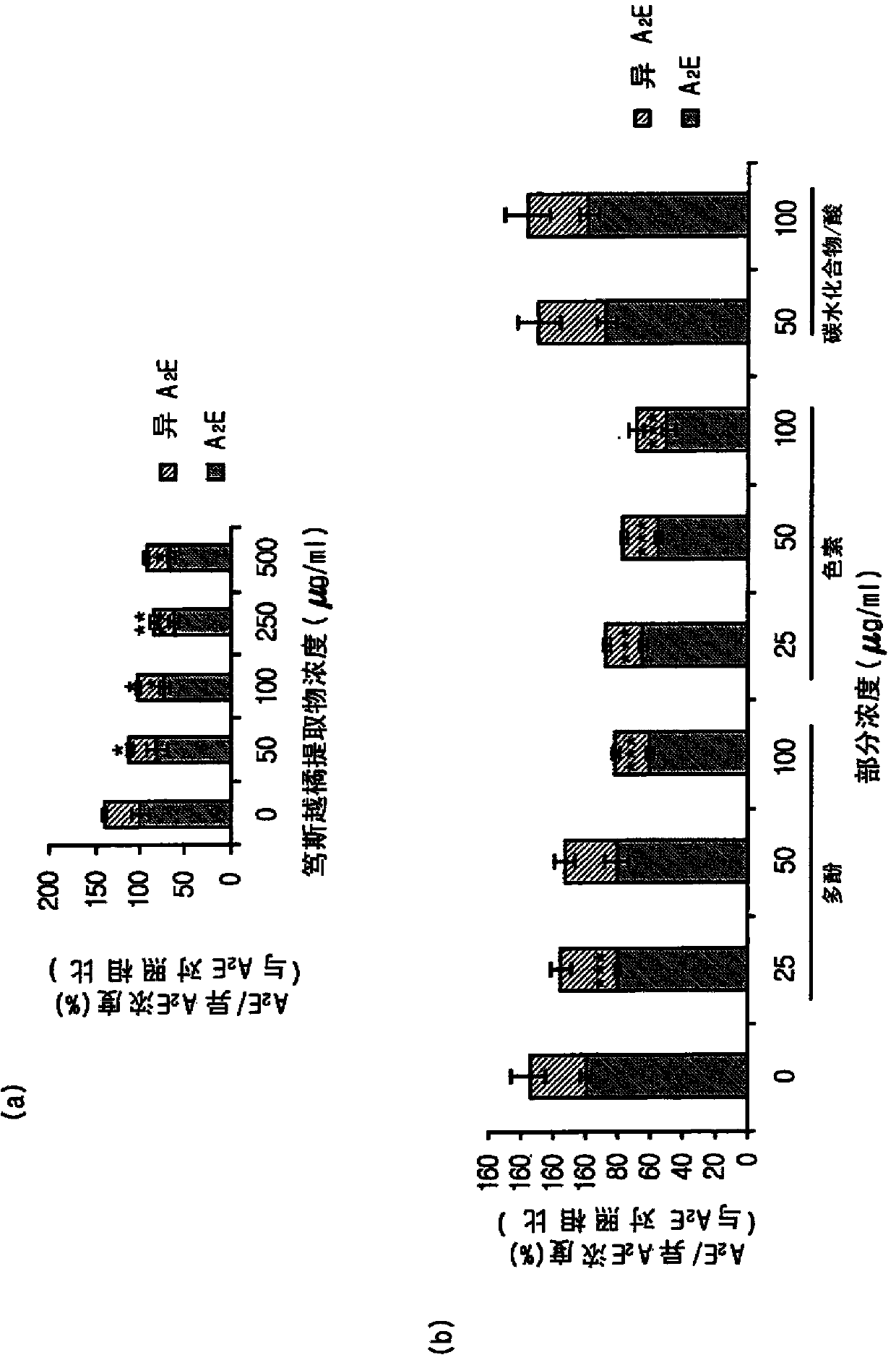
Composition for treating, preventing or relieving macular degeneration, containing vaccinium uliginosum extract or vaccinium uliginosum fractions as active ingredient
A technology of bilberry, macular degeneration, applied in the field of compositions for treating, preventing or improving macular degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of Bilberry Extract
[0046] Fruits of well-shaped lingonberries are selected and washed. 2,000 mL of water was added to 100 g of fruit, followed by two rounds of hot water extraction at 90° C. for 16 hours to obtain 10% bilberry extract. This bilberry extract was freeze-dried, processed into a powder and stored at -40°C until used to prepare the bilberry fraction or for the experiments below.
Embodiment 2
[0047] Example 2: Preparation of lingonberry fractions
[0048] The powdered Vaccinium extract prepared in Example 1 was dissolved in distilled water. Separately, two C18Sep-Pak cartridges were interconnected and preconditioned with 10 mL of ethyl acetate and anhydrous methanol, then 0.01N aqueous HCl was passed through the cartridges.
[0049] Load the extract solution into the cartridge, and then use 6 mL of 0.01N HCl aqueous solution to elute carbohydrates, acids, and other water-soluble compounds. The eluate was collected as a carbohydrate / acid fraction, which was used as a comparison in subsequent experiments.
[0050] Then, the attached C18Sep-Pak cartridge was purged with nitrogen for 10 min to dry the above cartridge. Then, 20 mL of ethyl acetate was passed through the dry cartridge to elute the phenolic compound and collect the polyphenyl fraction in a test tube.
[0051] Then, anthocyanins were eluted with 10 mL of anhydrous methanol containing 0.1% HCl to obtain ...
Embodiment 3
[0054] Example 3: Cell Culture
[0055] Human adult ARPE cells (ARPE-19; number CRL-2302) used in the experiments and analyzes of the present invention were obtained from Vision Science Institute, Catholic University of Korea, School of Medicine. at 37°C and 5% CO 2 ARPE cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U / ml penicillin and 100 mg / ml streptomycin in an atmospheric incubator. cells in 5 x 10 4 Cells / well were seeded in 6-well plates. Example 4: A2E Synthesis
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com