Pramipexole oral liquid and preparation method thereof
A technology of pramipexole and oral liquid, which is applied in the fields of pharmaceutical formulation, drug delivery, nervous system diseases, etc., and can solve the problems that the preparation of pramipexole oral liquid has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
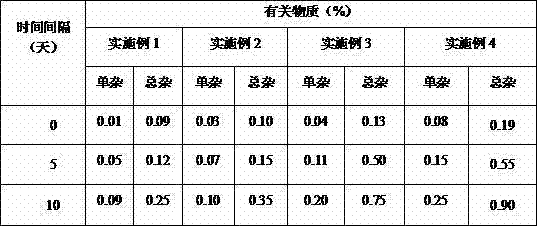
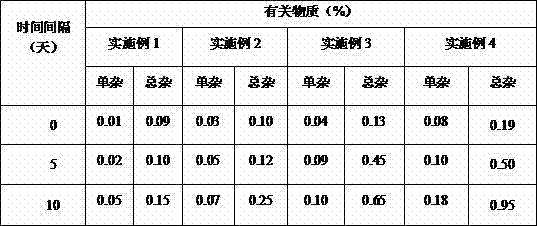
[0016] The pirfenidone tablet prepared by the present invention has a content of 0.2 g of pirfenidone per preparation unit. Specific examples are as follows:
[0017] Material name weight (g) Pramipexole dihydrochloride monohydrate 0.25 Sorbitol 200 citric acid 1.4 sodium citrate 0.7 Acesulfame K 3 sodium benzoate 2 water Appropriate amount
[0018] Preparation process: Weigh the prescribed amount of pramipexole dihydrochloride monohydrate, sorbitol, and acesulfame potassium into appropriate amount of water, add sodium benzoate after dissolving, finally add citric acid, sodium citrate, stir until clear and transparent, and constant volume , filtered and filled in brown plastic bottles.
[0019] Material name weight (g) Pramipexole dihydrochloride monohydrate 0.5 sucrose 200 Sodium dihydrogen phosphate 8.24 sodium hydroxide Appropriate amount Potassium sorbate 2 steviosid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com