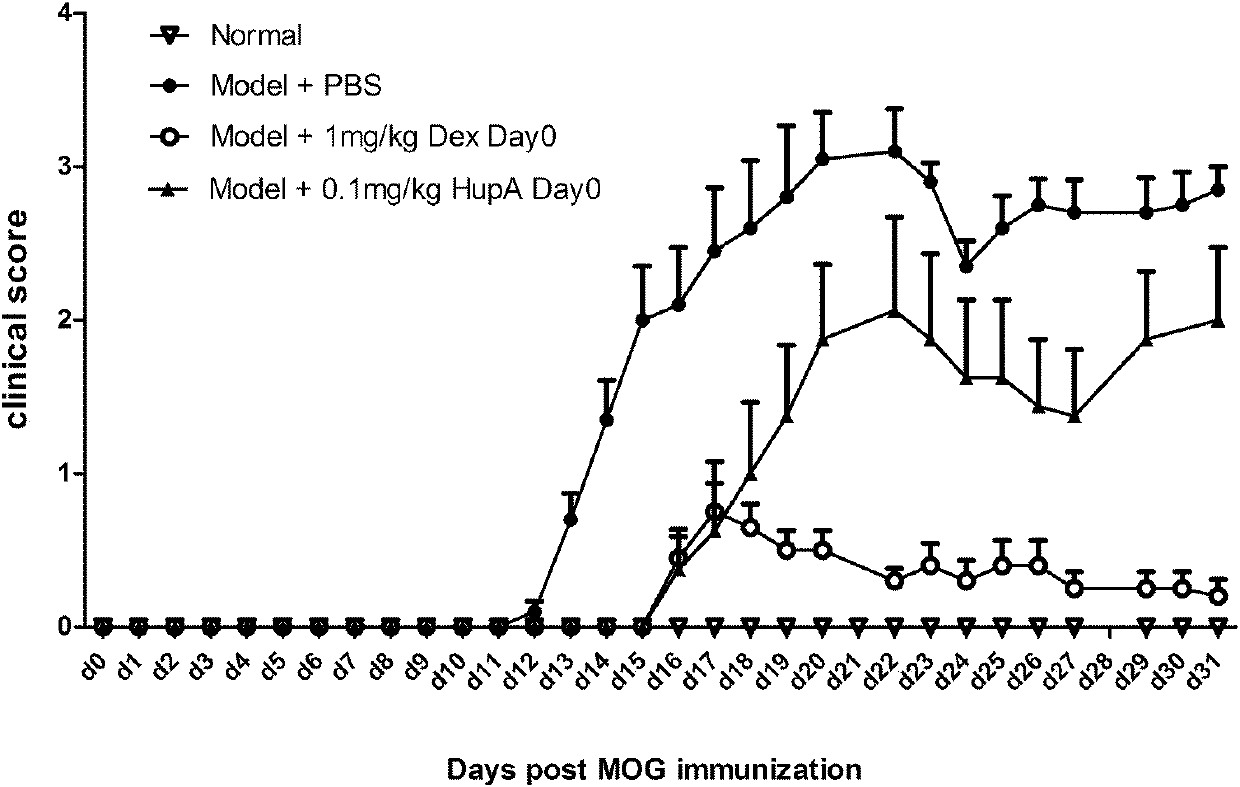
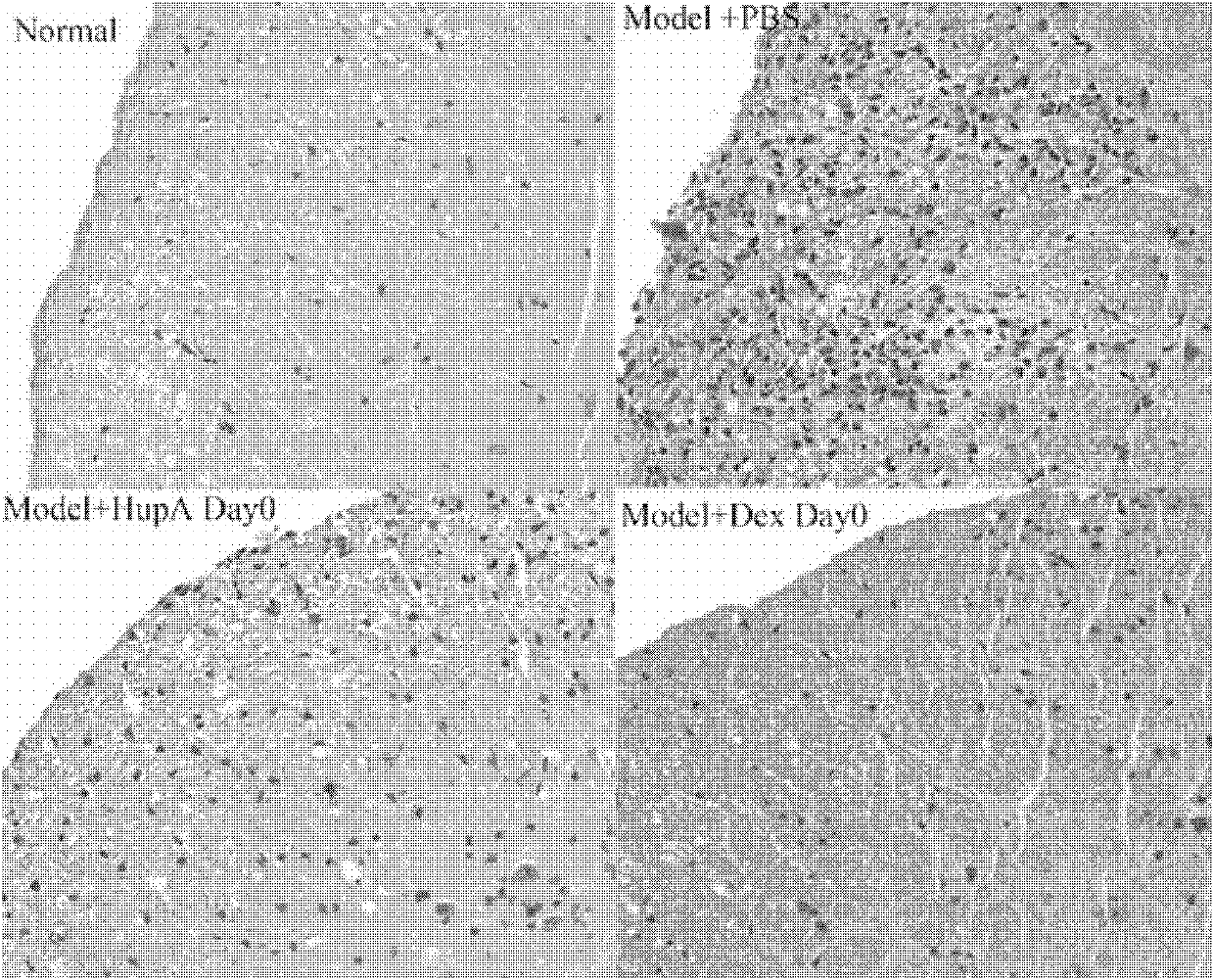
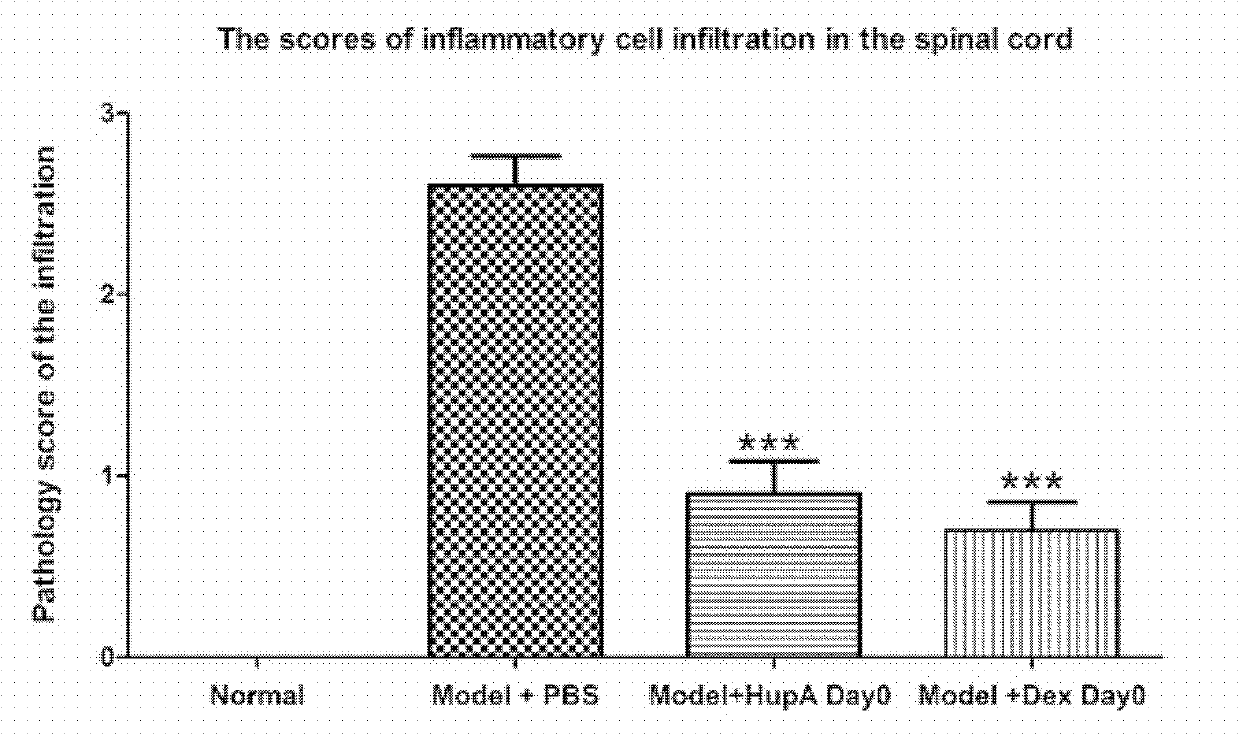
Application of Huperzine A in preparation of medicines preventing and treating multiple sclerosis disease
A technology of multiple sclerosis and huperzine A, applied in allergic diseases, drug combinations, nervous system diseases, etc., can solve problems such as the efficacy and application of Huperzine A that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Experimental animals:
[0039] A total of 40 SPF-grade female C57BL / 6 mice were used in this experiment, aged 8 weeks, weighing 16-20 g (Experimental Animal Center, Chinese Academy of Sciences). The feeding conditions are clean, 5-6 / cage, with random water supply; during the experiment period, paralyzed and endangered mice above grade 3 were fed in single cages, and the rat food was soaked in feeding water before being fed; the experimental animals were grouped completely randomly.
[0040] 2. EAE model preparation:
[0041] (1) Emulsifier preparation: oligodendrocyte glycoprotein polypeptide fragment-MOG 35-55 (artificially synthesized by Shanghai Huada Tianyuan Company, HPLC grade, purity 95%), complete Freund's Adjuvant (Complete Freund's Adjuvant, CFA, Sigma Company, F5881), BCG (60mg / support, Beijing Institute of Biological Products) fully emulsified Formulated as a white viscous water-in-oil formulation; each mouse requires 0.2ml of emulsifier containing 300u...
Embodiment 2
[0070] 1. Experimental animals:
[0071] A total of 40 SPF-grade female C57BL / 6 mice were used in this experiment, aged 8 weeks, weighing 16-20 g (Experimental Animal Center, Chinese Academy of Sciences). The feeding conditions are clean level, 5-6 birds / cage, free water supply. During the experiment period, the paralyzed and endangered mice above grade 3 were fed in single cages, and the rat food was soaked in feeding water before being fed; the experimental animals were divided into groups by a completely random method.
[0072] 2. EAE model preparation:
[0073] (1) Emulsifier preparation: oligodendrocyte glycoprotein polypeptide fragment-MOG 35-55 (artificially synthesized by Shanghai Huada Tianyuan Company, HPLC grade, purity 95%), complete Freund's Adjuvant (Complete Freund's Adjuvant, CFA, Sigma Company, F5881), BCG (60mg / support, Beijing Institute of Biological Products) fully emulsified Dubbed into a white viscous water-in-oil agent. Each mouse requires 0.2ml of e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com