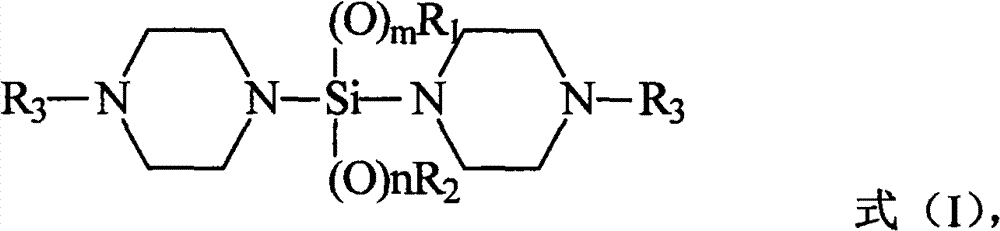
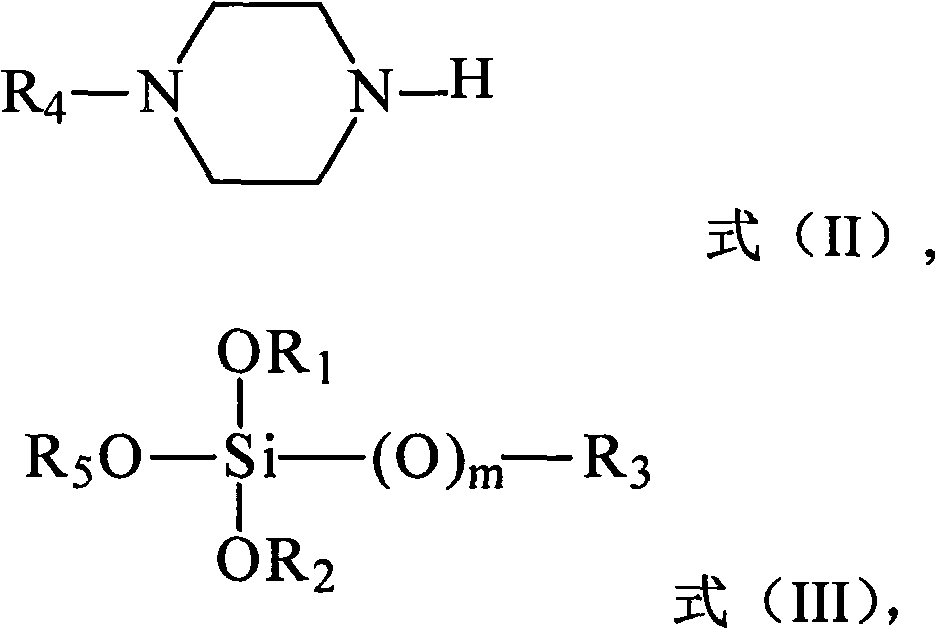
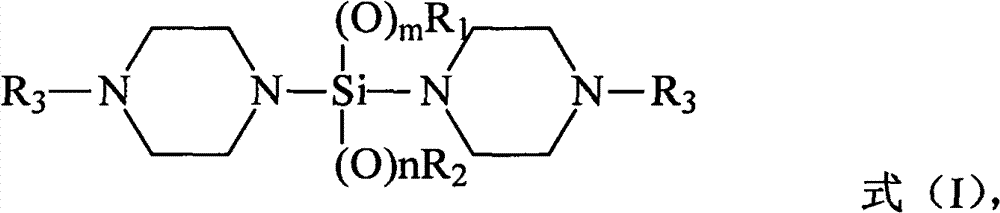A kind of silane compound containing oxygen and nitrogen heterocycle, its preparation method and application, and olefin polymerization method
A technology for siloxane compound and olefin polymerization, applied in the field of olefin polymerization, can solve the problems of harsh operating conditions, explosion, bumping, etc., and achieve the effects of low product cost, complete reaction and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0052] According to a preferred embodiment of the present invention, the preparation method of the silane compound containing oxygen nitrogen heterocyclic ring adopts the following steps: 2 Under protective conditions, in an aprotic solvent, add (N-alkyl)piperazine to the reaction flask in sequence, and stir evenly; under the condition of preferably 0-10°C, add n-butyllithium-n-hexane solution dropwise , the n-butyllithium-n-hexane solution is a commercially available reagent; then tetraalkoxysilane or alkyl trialkoxysilane is added dropwise; then the reaction solution is centrifuged and washed with the above aprotic solvent Once, the filtrate was collected, and the above-mentioned aprotic solvent was evaporated with a rotary evaporator, distilled under reduced pressure, and fractions were collected.
[0053] Since the olefin polymerization method provided by the invention mainly relates to the improvement of the external electron donor compound therein, that is, the silane co...
Embodiment 1
[0059] Prepare two (N-methyl) piperazinyl dimethoxysilanes according to the present invention in N 2 Under protective conditions, add 4.6g N-methylpiperazine and 30ml n-hexane to the reaction flask successively, and stir; at a temperature of 5°C, add 16.6ml (2.87M) butyllithium-n-hexane solution dropwise, The dropping time is 30 minutes; after 30 minutes, 3.47 g of tetramethoxysilane is added dropwise to the reaction solution, and the reaction is continued for 17 hours; the reaction solution is separated by centrifugation, the precipitate is washed twice with n-hexane, and the filtrate is collected; the n-hexane is evaporated with a rotary evaporator Alkanes solvent, vacuum distillation, collect 128-130 ℃ / 60Pa fraction, weigh 3.1g, carry out nuclear magnetic resonance test, get the following spectrum peak:
[0060] 1 HNMR (CDCl 3 / TMS, 300MHz) δ (ppm): 2.27 (s+m, 14H, 4NCH 2 , 2NCH 3 ), 3.01(m, 8H, 4CH 2 ), 3.45(s, 6H, 2OCH 3 )
Embodiment 2
[0062] Preparation of bis(N-methyl)piperazinylmethylmethoxysilane according to the invention
[0063] in N 2 Under protective conditions, add 3.6g N-methylpiperazine and 40ml n-hexane to the reaction flask in turn, and stir; at a temperature of 10°C, add 13ml (2.9M) butyllithium-n-hexane solution dropwise, dropwise The time is 40 minutes; after 20 minutes, 2.5 g of methyltrimethoxysilane is added dropwise to the reaction solution, and the reaction is continued for 17 hours; the reaction solution is separated by centrifugation, the precipitate is washed twice with n-hexane, and the filtrate is collected; the n-hexane is evaporated with a rotary evaporator The solvent was distilled under reduced pressure, and the fraction at 122-128°C / 110Pa was collected, weighing 2.4g, and tested by nuclear magnetic resonance, and the following peaks were obtained:
[0064] 1 HNMR (CDCl 3 / TMS, 300MHz) δ (ppm): 0.07 (s, 3H, CH 3 ), 2.27 (s+m, 14H, 4NCH 2 , 2NCH 3 ), 2.96(t, 8H, 4CH 2 ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com