Electrochemical determination method of stabilizer in chemical nickel plating solution
A technology of electroless nickel plating solution and measurement method, which is applied in the field of electrochemical measurement to achieve the effect of good reproducibility and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
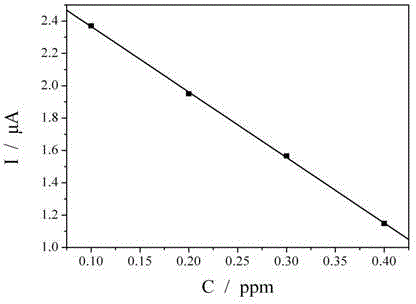
[0017] The mensuration of sodium tetrathionate in the electroless nickel plating liquid of embodiment 1
[0018] Put the clean gold electrode (diameter 2mm) in the electroless nickel plating solution containing sodium tetrathionate at 25°C for 15 minutes, wash the surface of the gold electrode with deionized water, and place the gold electrode in a solution containing 5.0x10 -4 moL -1 K 3 Fe(CN) 6 and 0.1molL -1 In the solution of KCl, a saturated calomel electrode is used as a reference electrode, and a platinum wire is used as a counter electrode to form a three-electrode system. At 25°C, K 3 Fe(CN) 6 Revert to K 4 Fe(CN) 6 current.
[0019] Prepare standard solutions with sodium tetrathionate concentrations of 0, 0.1, 0.2, 0.3 and 0.4 ppm respectively. Similarly, put the clean gold electrodes (diameter 2mm) in the above standard solution of sodium tetrathionate at 25°C for 15 minutes, wash the surface of the gold electrodes with deionized water, and place the go...
Embodiment 2
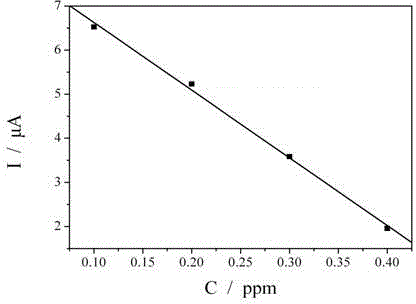
[0021] The mensuration of thiourea in the electroless nickel plating liquid of embodiment 2
[0022] Put the clean gold electrode (diameter 2mm) in the electroless nickel plating solution containing thiourea at 25°C for 15 minutes, wash the surface of the gold electrode with deionized water, and place the gold electrode in a solution containing 5.0x10 -4 moL -1 K 3 Fe(CN) 6 and 0.1molL -1 In the solution of KCl, a saturated calomel electrode is used as a reference electrode, and a platinum wire is used as a counter electrode to form a three-electrode system. At 25°C, K 3 Fe(CN) 6 Revert to K 4 Fe(CN) 6 current.
[0023] Prepare standard solutions with thiourea concentrations of 0, 0.1, 0.2, 0.3 and 0.4 ppm respectively. Similarly, put clean gold electrodes (diameter 2mm) in the above-mentioned standard solution of thiourea at 25°C for 15 minutes, wash the surface of the gold electrodes with deionized water, and place the gold electrodes in a solution containing 5.0...
Embodiment 3
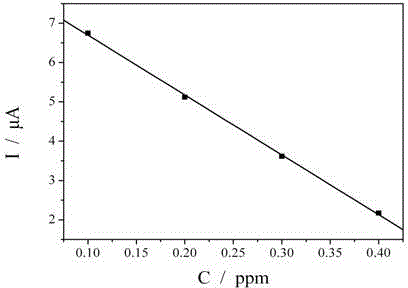
[0025] The mensuration of cysteine in the electroless nickel plating liquid of embodiment 3
[0026]Put the clean gold electrode (diameter 2mm) in the electroless nickel plating solution containing cysteine at 25°C for 15 minutes, wash the surface of the gold electrode with deionized water, and place the gold electrode in a solution containing 5.0x10 -4 moL -1 K 3 Fe(CN) 6 and 0.1molL -1 In the solution of KCl, a saturated calomel electrode is used as a reference electrode, and a platinum wire is used as a counter electrode to form a three-electrode system. At 25°C, K 3 Fe(CN) 6 Revert to K 4 Fe(CN) 6 current.
[0027] Prepare standard solutions with cysteine concentrations of 0, 0.1, 0.2, 0.3 and 0.4 ppm respectively. Similarly, put the clean gold electrodes (diameter 2mm) in the above-mentioned cysteine standard solution at 25°C for 15 minutes, wash the surface of the gold electrodes with deionized water, and place the gold electrodes in the solution cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com