Anti-tumor 2-amino nicotinonitrile, application and preparation method thereof
A technology for anti-tumor drugs and compounds, applied in the field of medicine, can solve problems such as poor selectivity, toxic and side effects, and achieve the effects of rapid response, high selectivity, and growth inhibition in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 4-(4-Hydroxy-3,5-dimethoxyphenyl)-6-phenyl-2-aminonicotinonitrile (a) (W11)
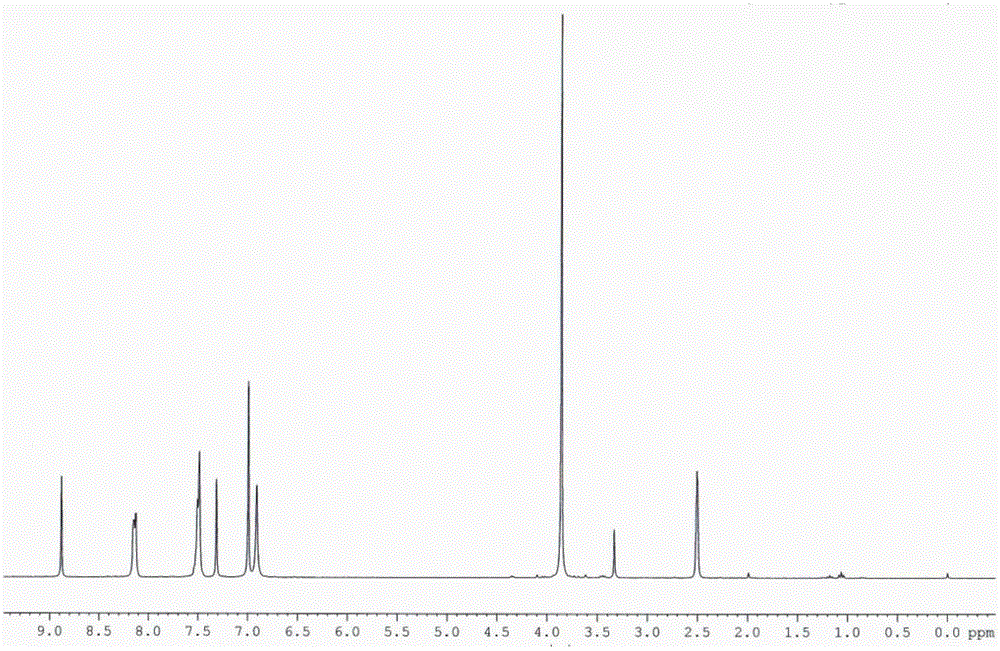
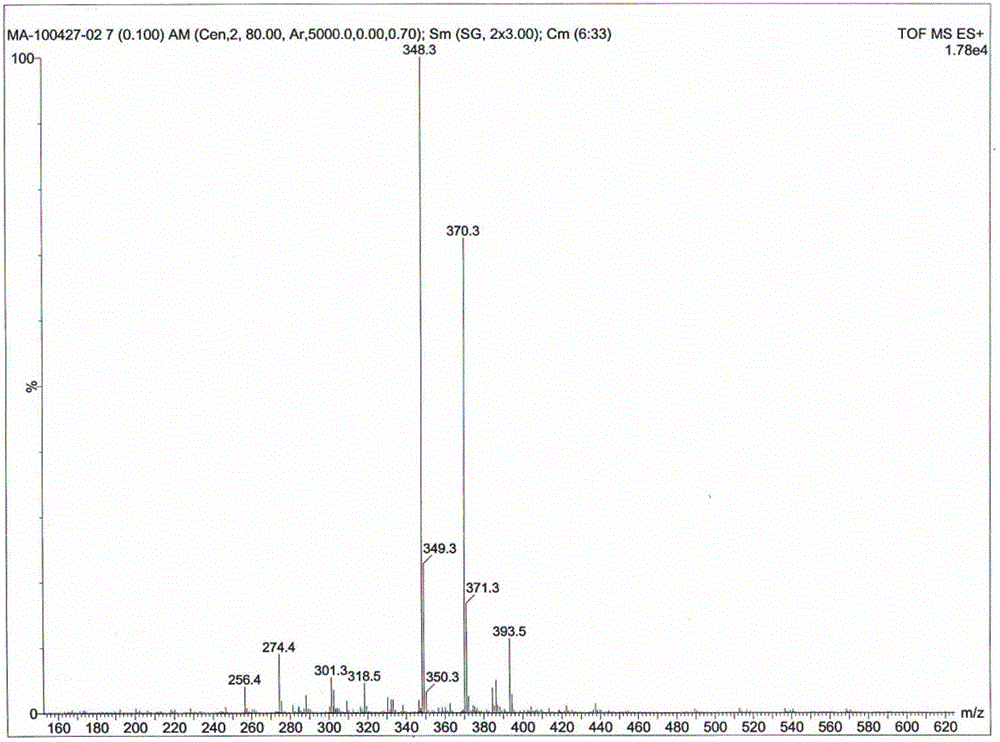
[0029] Dissolve syringaldehyde (2 mmol), acetophenone (2 mmol) and malononitrile (2 mmol) in 1,4-dioxane, then add ammonium acetate (4 mmol), microwave (300 W) Auxiliary heating at 120°C for 20 min. Ice water was added to the reaction mixture to precipitate the product, and then recrystallized to obtain the product with a yield of 84%. IR (KBr) v / cm -1 : 3471, 3395, 3358, 3065, 2953, 2111, 1630, 1573, 1588, 1514, 1464, 1379, 1250, 1211, 1109, 770, 699; 1 H NMR (300 MHz, DMSO-d 6 ) δ / ppm: 8.88 (s, 1H, OH), 8.12-8.15 (m, 2H, ArH), 7.45-7.56 (m, 3H, ArH), 7.32 (s, 1H, H5), 7.00 (s, 2H , ArH), 6.91 (s, 2H, NH 2 ), 3.86 (s, 6H, 2×OCH 3 ); 13 C NMR (75.5 MHz, DMSO-d 6 ) δ / ppm: 160.9, 158.3, 155.0, 147.9, 137.7, 137.3, 130.0, 128.5, 127.2, 126.6, 117.5, 109.1, 106.3, 86.5, 56.2; MS (ESI) m / z: 348.3 + , 370.3 [M+Na] + ; Anal. Calcd for C 20 h 17 N 3 o 3 : C, 69.15; H, 4.93; N...
Embodiment 2
[0031]
[0032] 4-(4-Hydroxy-3-methoxyphenyl)-6-phenyl-2-aminonicotinonitrile (b) (W12)
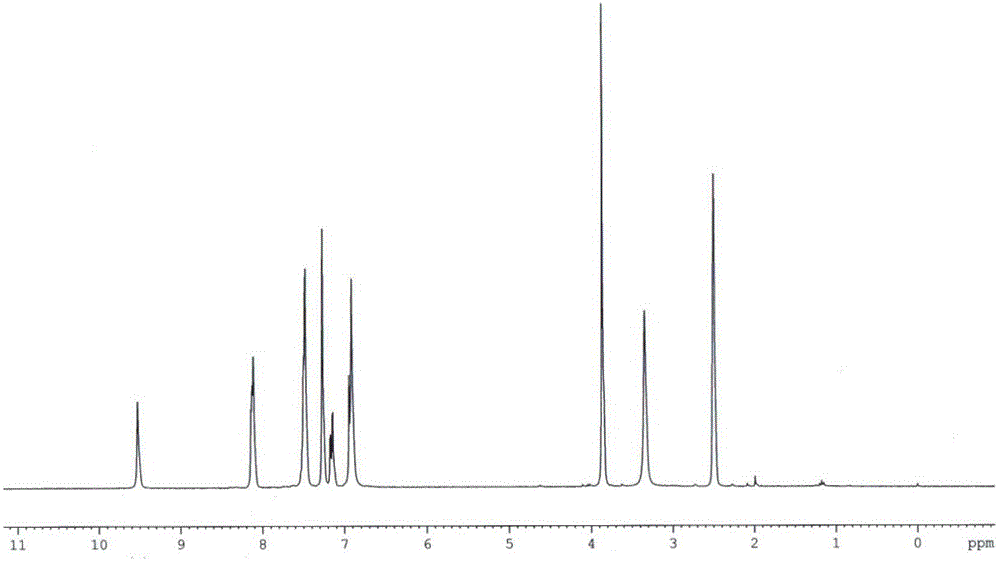
[0033] Dissolve vanillin (2 mmol), acetophenone (2 mmol), and malononitrile (2 mmol) in 1,4-dioxane, then add ammonium acetate (4 mmol), microwave (300 W) Auxiliary heating at 120°C for 20 min. Ice water was added to the reaction mixture to precipitate the product, and then recrystallized to obtain the product with a yield of 82%. IR (KBr) v / cm -1 : 3486, 3401, 3308, 3196, 2209, 1632, 1577, 1551, 1519, 1469, 1278, 1220, 1126, 764; 1 H NMR (300 MHz, DMSO-d 6 ) δ / ppm: 9.53 (s, 1H, OH), 8.11-8.14 (m, 2H, ArH), 7.42-7.56 (m, 3H, ArH), 7.24-7.29 (m, 2H, H5, ArH), 7.15 (dd, 1H, ArH, J = 2.1, 8.1 Hz), 6.85-6.96 (m, 3H, ArH, NH 2 ), 3.87 (s, 3H, OCH 3 );13 C NMR (75.5 MHz, DMSO-d 6 ) δ / ppm: 161.0, 158.3, 154.9, 148.2, 147.5, 137.7, 129.9, 128.6, 127.7, 121.4, 117.5, 115.5, 112.6, 109.0, 86.4, 55.8 [MS (ESI 3H.8) m / z ] + , 340.3 [M+Na] + , 356.0 [M+K] + ; Anal. Calcd for C 19 h 15 ...
Embodiment 3
[0034] Embodiment 3 Pharmacological experiments related to (W11) and (W12)
[0035] 1. Screening for anti-tumor activity in vitro
[0036] 1.1 Test substance
[0037] The test substances are the compounds prepared in Example 1 and Example 2 of the present invention.
[0038] a(W11) is the compound prepared in Example 1 of the present invention, the Chinese name is 4-(4-hydroxy-3,5-dimethoxyphenyl)-6-phenyl-2-aminonicotinonitrile; b(W12 ) is the compound prepared in Example 2 of the present invention, and its Chinese name is 4-(4-hydroxy-3,5-dimethoxyphenyl)-6-phenyl-2-aminonicotinonitrile. The positive drug is doxorubicin, purchased from Sigma.
[0039] 1.2 Tested cell lines
[0040] Human gastric cancer SGC7901, human liver cancer HepG2, human leukemia cell line HKB, human pancreatic cancer cell line KP4, and human lung cancer cell lines NCI-H292 and NCI-H727 were selected.
[0041] 1.3 Experimental method
[0042] Take a bottle of cells in a good state in the expon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com