New application of curcumin
A technology of curcumin and demethoxycurcumin, which is applied in the field of curcumin, can solve the problems of limited application of organ toxicity, adverse consequences, and acid-base balance disruption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The above-mentioned content of the present invention will be described in further detail below through specific embodiments in the form of examples, but it should not be understood that the scope of the above-mentioned theme of the present invention is limited to the following examples. All technologies realized based on the above contents of the present invention belong to the scope of the present invention.
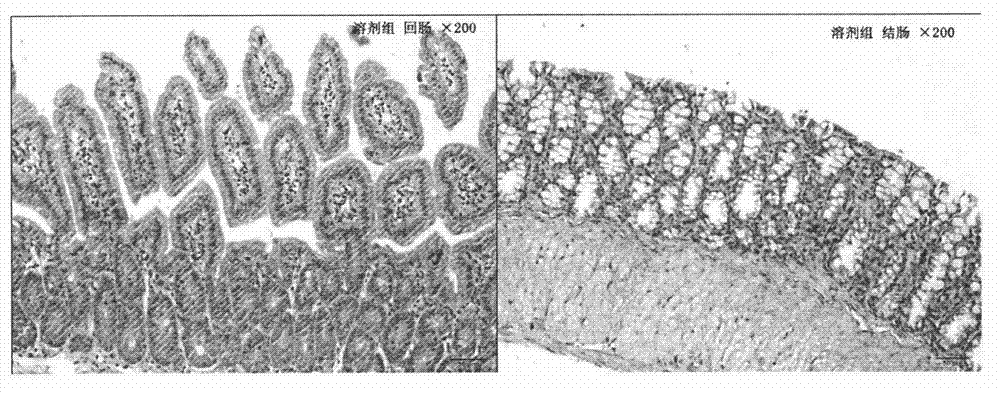
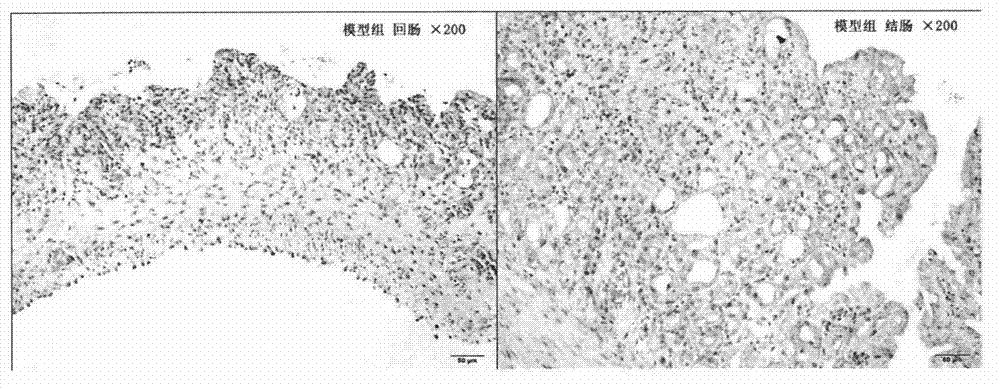
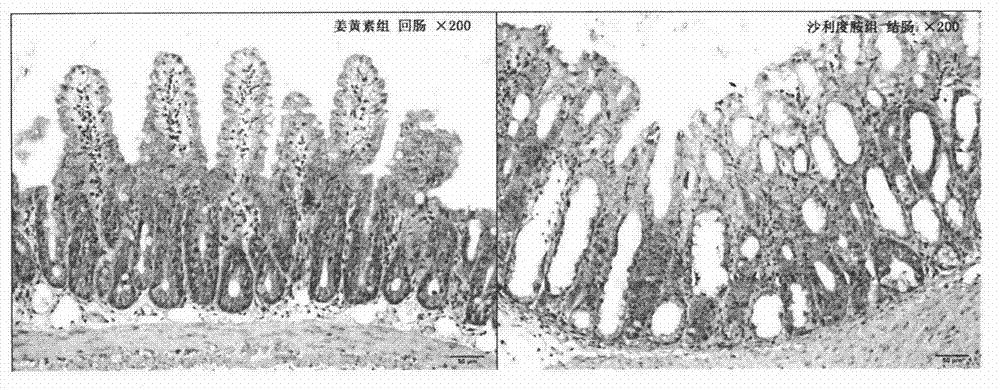
[0027] The therapeutic effect of embodiment curcumin on delayed diarrhea caused by irinotecan
[0028] 1. Experimental method
[0029] On the basis of the pre-test, 32 ICR male mice were randomly divided into 4 groups, with 8 mice in each group, and each mouse was housed in a single cage, with free access to food and water. The model group was intraperitoneally injected irinotecan 75mg / kg every day for four consecutive days; the curcumin group was intraperitoneally injected irinotecan 75mg / kg every day for four consecutive days, and at the same time intragastric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com