Preparation for restoring consciousness and calming nasal mucosa and preparation method thereof
A technology for nasal preparations, which is applied in the field of nasal preparations of pharmaceutical compositions for the treatment of cerebral apoplexy. It can solve the problems of increasing the incidence of adverse reactions and medication risks, high treatment costs, and delays in treatment, and achieves improved absorption, easy absorption, and Obvious effect of absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
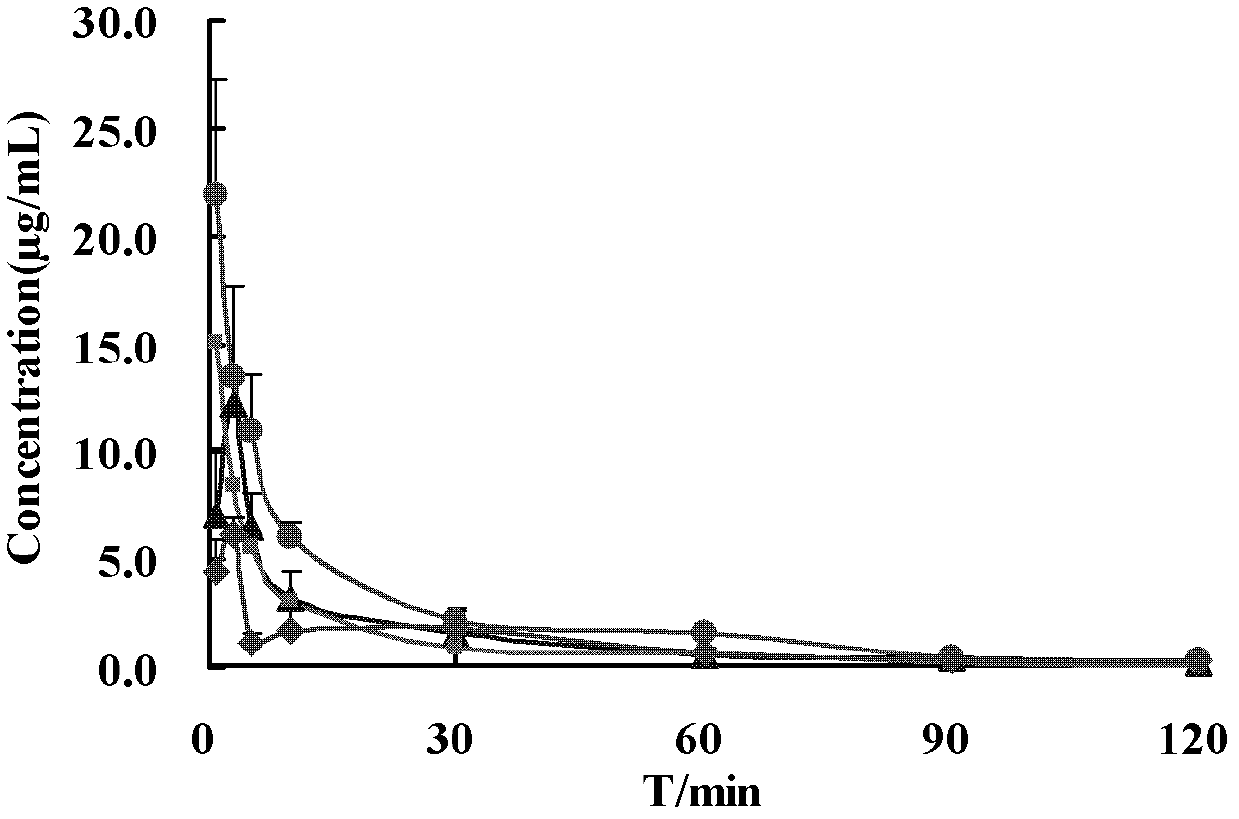
[0062] Experimental Example 1: Study on hemopharmacokinetics of the gardenia extract (prepared by the method of Example 1) in the pharmaceutical composition of the present invention combined with moxa tablets after nasal administration to rats
[0063] 25 male SD rats, weighing 250-300g, were randomly divided into 5 groups, 5 rats in each group, respectively Gardenia extract + high-concentration moxa nasal drops group (A), gardenia extract + medium-concentration Ai Pian nasal drops group (B), Gardenia extract + low concentration Ai Pian nasal drops group (C), Gardenia extract nasal drops group (D) and Gardenia extract + high concentration Ai Pian injection Group (E) fasted 12 hours before the experiment and had free access to water.
[0064] Rats in groups A, B, C, and D were given each nasal drop according to 0.1mL·kg-1 body weight (i.e. 12 mg / kg of Gardenia extract); E group was given 4 mL·kg-1 body weight (Gardenia extract 12mg / kg) tail vein injection.
[0065] Table 1: P...
experiment example 2
[0070] Experimental Example 2: Study on blood and brain pharmacokinetics of gardenia extract (prepared by the method in Example 1) in the pharmaceutical composition of the present invention combined with moxa tablet in mice after nasal administration
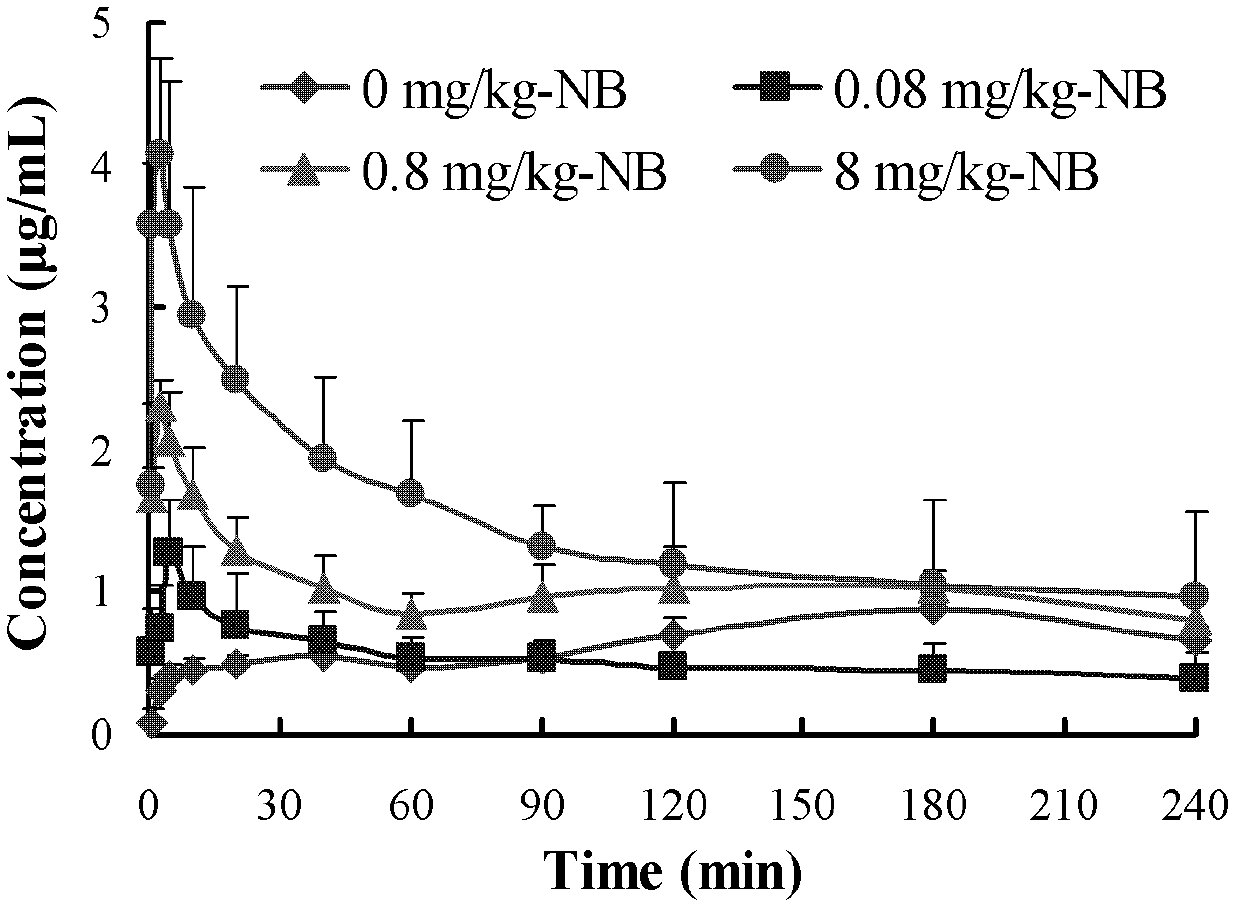
[0071] 160 male ICR mice, weighing 20-25g, were randomly divided into 4 groups, 40 in each group, respectively Gardenia extract + high concentration moxa nasal drops group (I), Gardenia extract + medium concentration Ai Pian nasal drops group (II), Gardenia extract + low concentration Ai Pian nasal drops group (III) and Gardenia extract nasal drops group (IV). They fasted 12 hours before the experiment and had free access to water.
[0072] Mice in groups (I), (II), (III) and (IV) were fed by 3 μL·20g -1 Body weight (ie gardenia extract 18mg / kg) was given each nasal drop.
[0073] Table 2: Hemopharmacokinetic parameters of mice in each group
[0074]
[0075] * P** P<0.01, vs Group i.v.
[0076] Depend on image 3 It ca...
experiment example 3
[0081] Experimental Example 3: Study on the pharmacokinetics of the gardenia extract (prepared by the method of Example 1) in the pharmaceutical composition of the present invention combined with moxa tablets administered to mice via different routes of administration
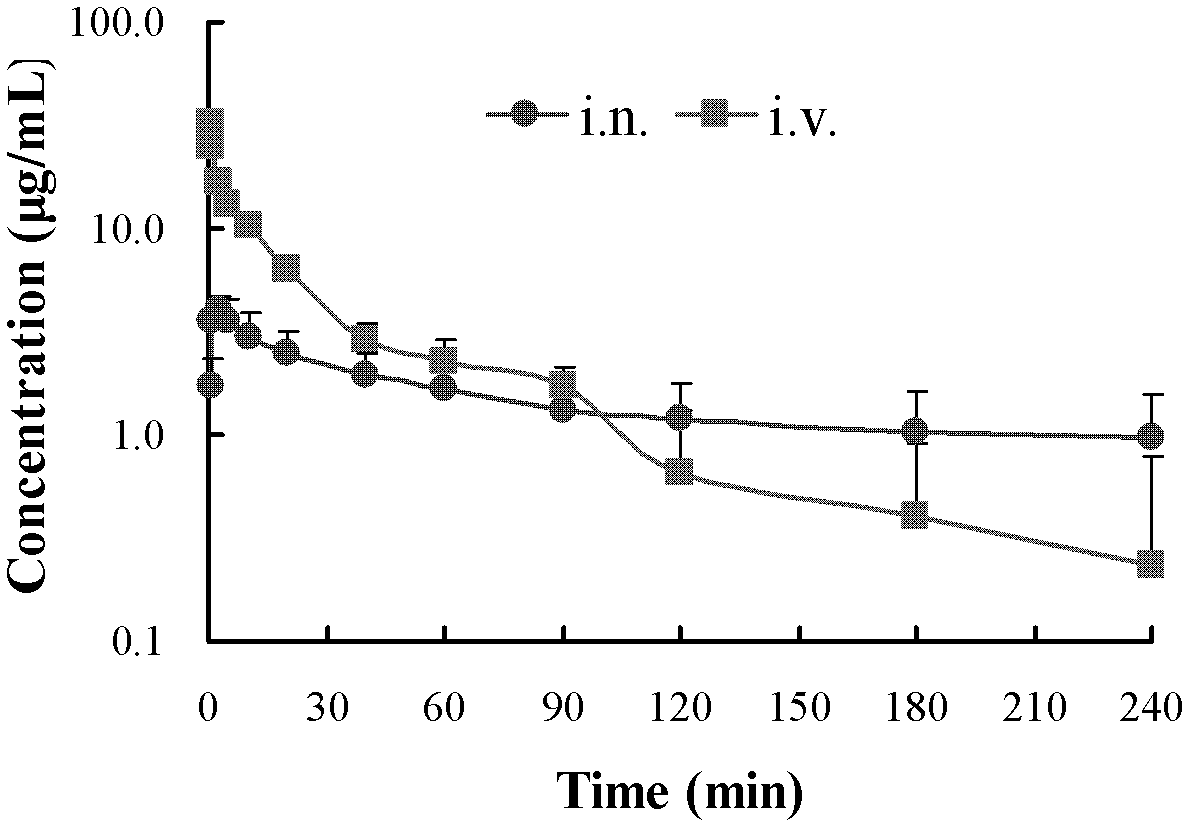
[0082] 120 male ICR mice, weighing 20-25g, were randomly divided into 3 groups, 40 in each group, respectively Gardenia extract + Ai Pian nasal drops group (i.n.), Gardenia extract + Ai Pian injection group (i.v.) and gardenia extract + moxa tablet oral solution group (p.o.), fasting 12h before the experiment, free to drink water.
[0083] Mice in groups (i.n.), (i.v.), and (p.o.) were given the preparations of each group through different routes, ie nasal cavity, injection and oral administration, at equal doses.
[0084] Table 4: Hemopharmacokinetic parameters of mice in each group
[0085]
[0086] * P** P<0.01, vs Group i.v.
[0087] Depend on Figure 5 The drug-time curve and the pharmacokinetic par...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com