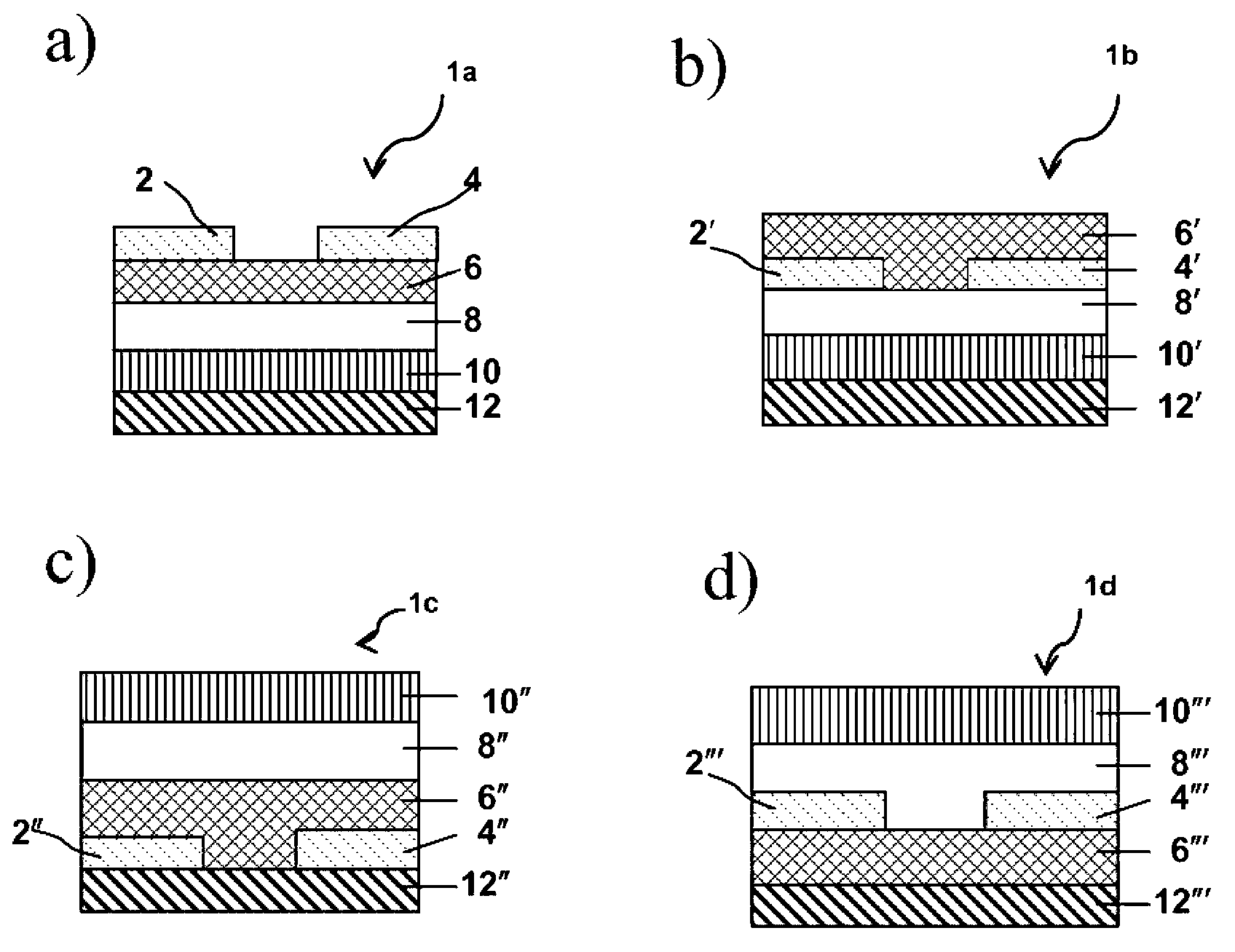
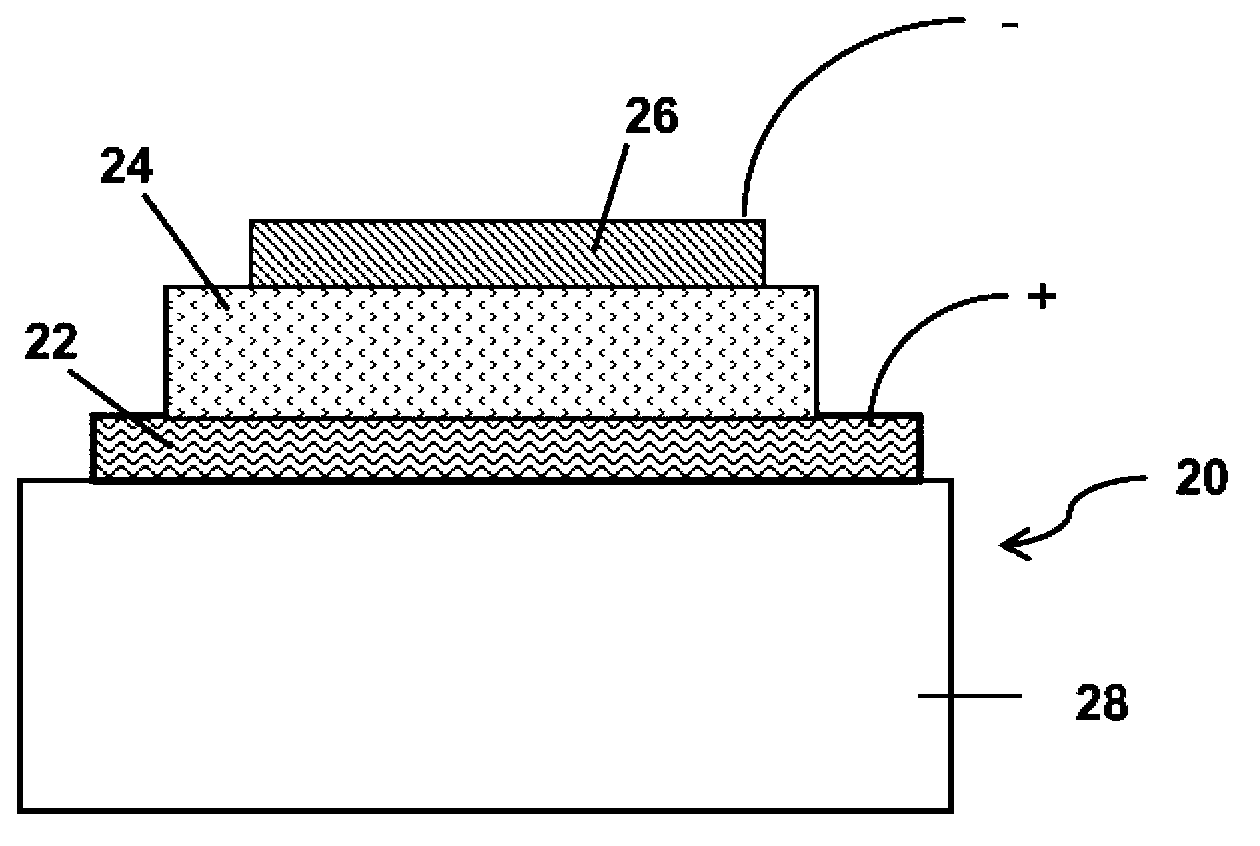
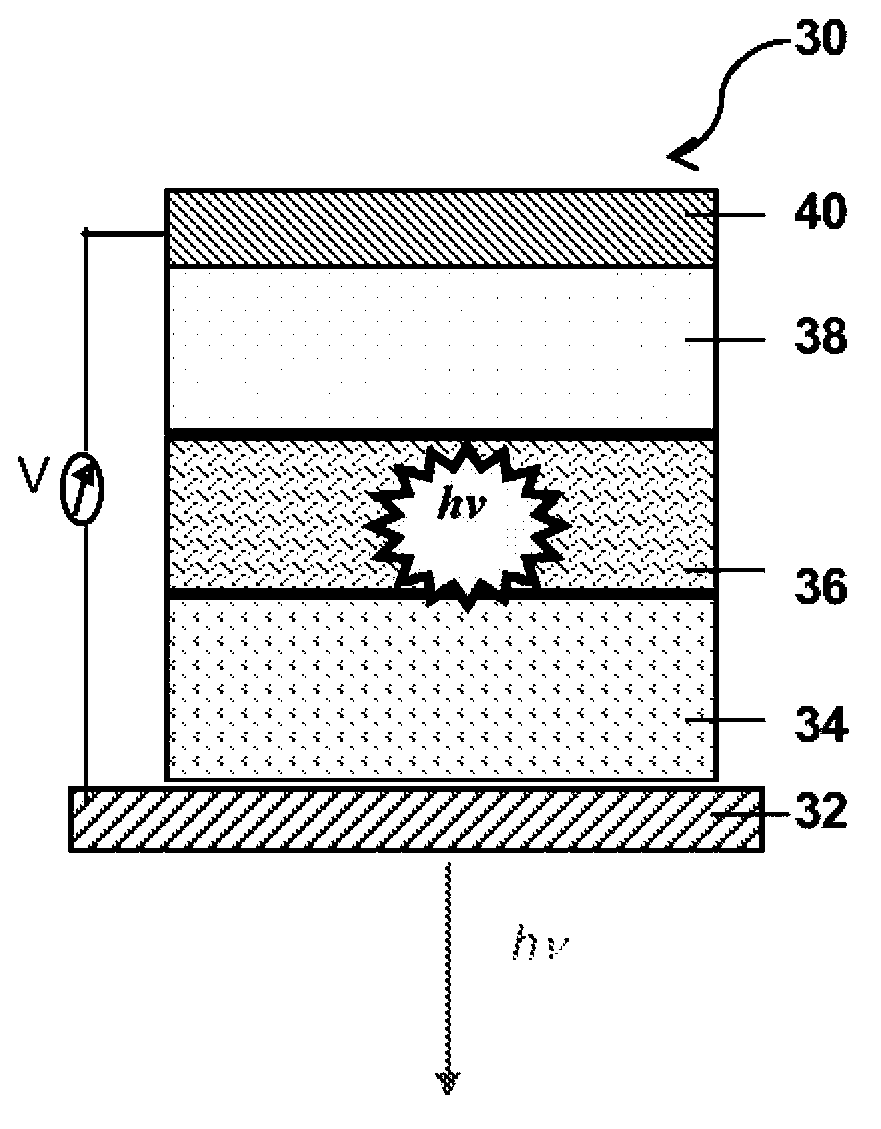
Thionated aromatic bisimides as organic semiconductors and devices incorporating them
A kind of substituent, halogenated alkyl technology, applied in the field of thiosulfuric acid aromatic diimide as organic semiconductor and devices using them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Embodiment 1: synthesis
Embodiment 1A
[0245] Example 1A: Preparation of (S, S)-PDI1MP, (S, S)-PDIS 1 1MP, (S,S)-trans-PDIS 2 1MP, (S,S)-cis-PDIS 2 1MP, (S, S)-PDIS 3 1MP and (S,S)-PDIS 4 1MP
[0246]
[0247]
[0248] 13.5g (34.4mmol) of perylene-3,4,9,10-tetracarboxylic dianhydride (Aldrich), 11.4g (113mmol) of (S)-(+)-2-aminohexane (Alfa Aesar, 99+% tolerance) and 130 g of imidazole were heated in a sealed container at 200°C for 2 hours. The mixture was cooled slightly and then treated with 700 mL of 1,4-dioxane. The mixture was stirred at ambient temperature for 2 hours then filtered. The filter cake was washed successively with methanol (100 mL) and diethyl ether (100 mL). The filter cake was dried to yield 17.9 g (93% yield) of (S,S)-PDI1MP as a dark red powder.
[0249] 1 H NMR (CDCl 3 ,500MHz):δ8.62(d,4H,J=8.0),8.51(d,4H,J=8.0),5.32(m,2H),2.25(m,2H),1.98(m,2H),1.67 (d,6H,J=6.9),1.44-1.24(m,8H),0.90(t,6H,J=7.1). 13 C NMR (CDCl 3 ,125MHz): δ163.8,134.3,131.2,129.3,12...
Embodiment 1B
[0259] Example 1B: Preparation of trans-PDIS 2 1MHex and cis-PDIS 2 1MHex (racemic and meso spin mixture)
[0260]
[0261]
[0262] 4.2g (10.7mmol) of perylene-3,4,9,10-tetracarboxylic dianhydride (Aldrich), 4.1g (35.6mmol) of (+ / -)-2-aminoheptane (Aldrich), and A mixture of 40 g of imidazole was heated at 200° C. for 2 hours in a sealed container. The mixture was cooled slightly and then triturated in 70 mL of 1,4-dioxane. The mixture was cooled to room temperature and filtered. The filter cake was refluxed with 180 mL of 1,4-dioxane for 10 minutes, cooled to room temperature, filtered, and then successively washed with 100 mL of methanol and twice with 80 mL of diethyl ether. The filter cake was dried to yield 4.8 g (76% yield) of PDIIMHex as a bright red powder.
[0263] 1 H NMR (CDCl 3 ,500MHz):δ8.68(d,4H,J=8.0),8.63(d,4H,J=8.0),5.30(m,2H),2.23(m,2H),1.94(m,2H),1.61 (d,6H,J=7.5),1.42-1.20(m,12H),0.85(t,6H,J=8.0).C 38 h 38 N 2 o 4 The analytic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com