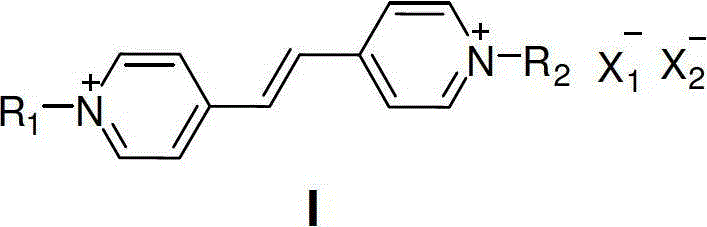
Conjugated expanded viologen derivative as well as preparation method and application thereof
A derivative, viologen technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of limited application range, difficulty in making electrochromic devices, monotonous display colors, etc., and achieve low cost and good performance. Electrochromic performance, convenient post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0028]
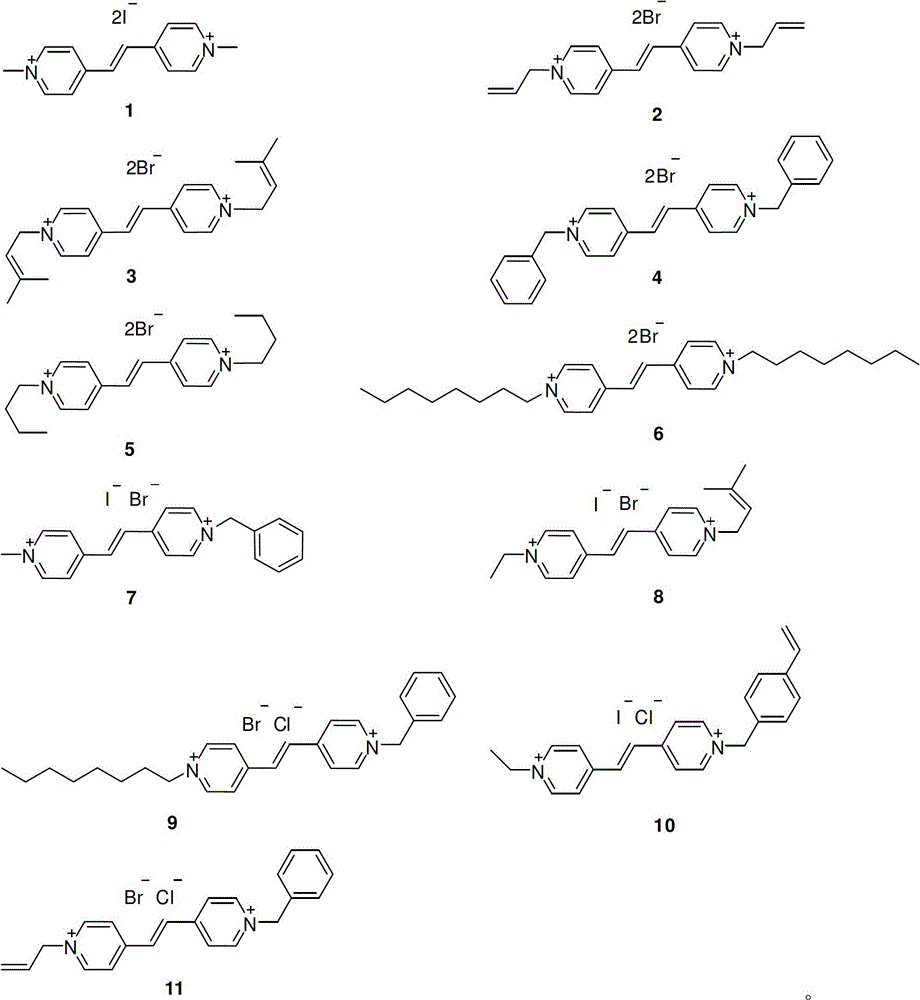
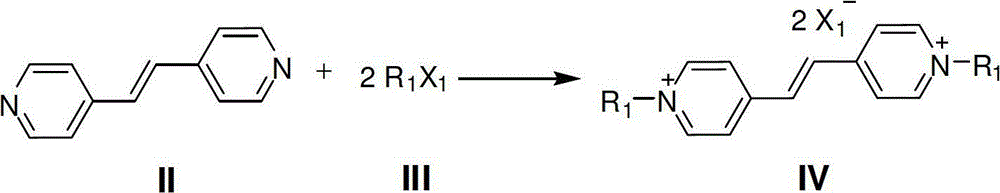
[0029] Add 18.2g (0.1mol) of 1,2-bis(4-pyridyl)ethylene into 150ml of acetonitrile, then slowly drop into 35.5g (0.25mol) of methyl iodide, stir at 30°C for 72h, add twice the volume of diethyl ether As a precipitating agent, filter, wash the filter cake with anhydrous ether, and dry at 50°C to obtain N,N'-dimethyl-1,2-bis(4-pyridyl)ethylene bis-quaternary ammonium iodide 1( Orange-red solid), the yield is 82%; the structure of the product is identified to be completely consistent with the above structural formula 1.
preparation Embodiment 2
[0031]
[0032] Add 18.2g (0.1mol) of 1,2-bis(4-pyridyl)ethylene into 150ml of acetonitrile, then slowly drop in 27.2g (0.225mol) of allyl bromide, raise the temperature to 50°C and stir for 72h, then cool to At room temperature, add twice the volume of diethyl ether as a precipitant, filter, wash the filter cake with anhydrous diethyl ether, and dry at 50°C to obtain N,N'-diallyl-1,2-bis(4-pyridyl) Ethylene bis-quaternary ammonium bromide 2 (light yellow solid), the yield is 83%; the structure of the product is identified to be completely consistent with the above structural formula 2.
preparation Embodiment 3
[0034]
[0035] Add 18.2g (0.1mol) of 1,2-bis(4-pyridyl)ethylene into 150ml of acetonitrile, then slowly drop into 33.6g (0.225mol) of bromoisoamylene, heat up to 80°C and reflux for 60h, then cool to At room temperature, add twice the volume of diethyl ether as a precipitant, filter, wash the filter cake with anhydrous diethyl ether, and dry at 50°C to obtain N,N'-diprenyl-1,2-bis(4-pyridyl ) Ethylene bisquaternary ammonium bromide 3 (light yellow solid), the yield is 85%; the structure of the product is identified to be completely consistent with the above structural formula 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com