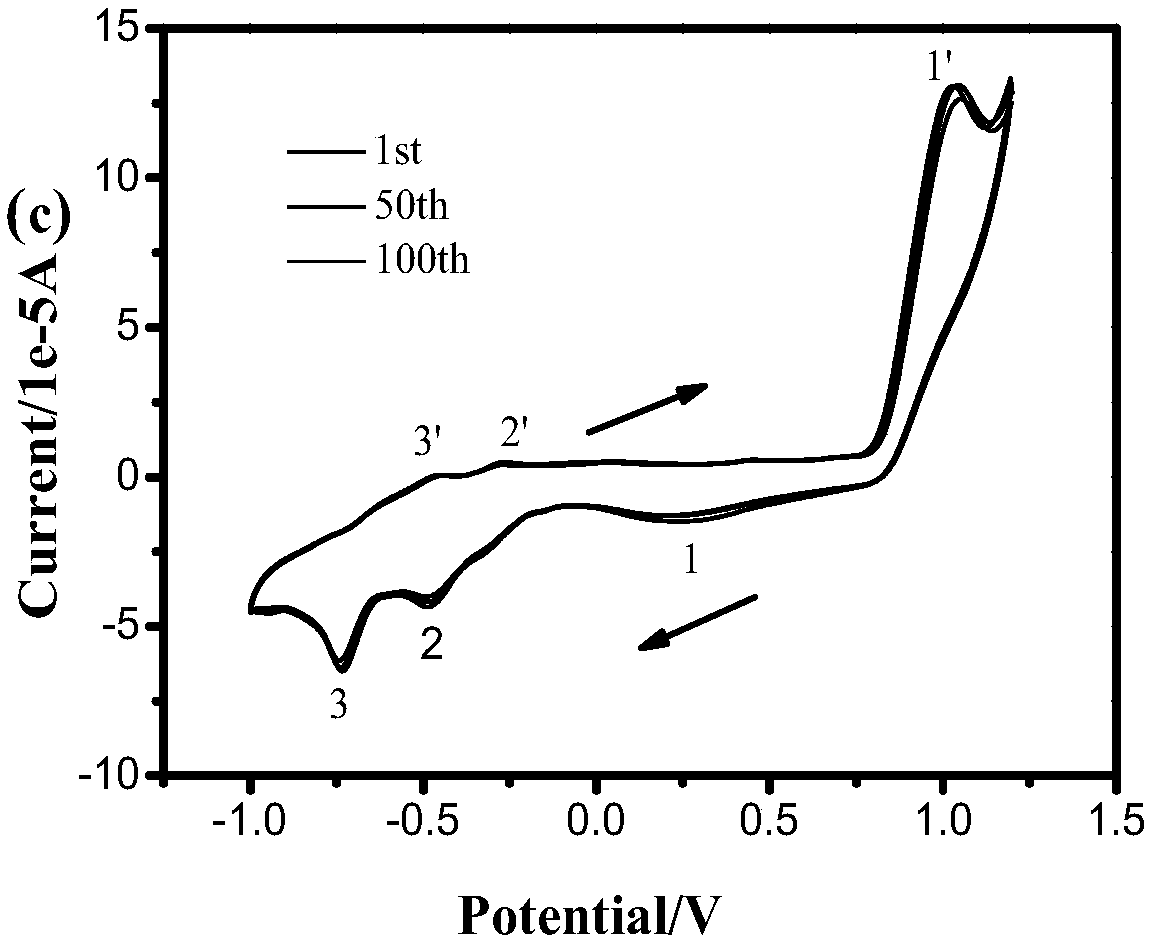
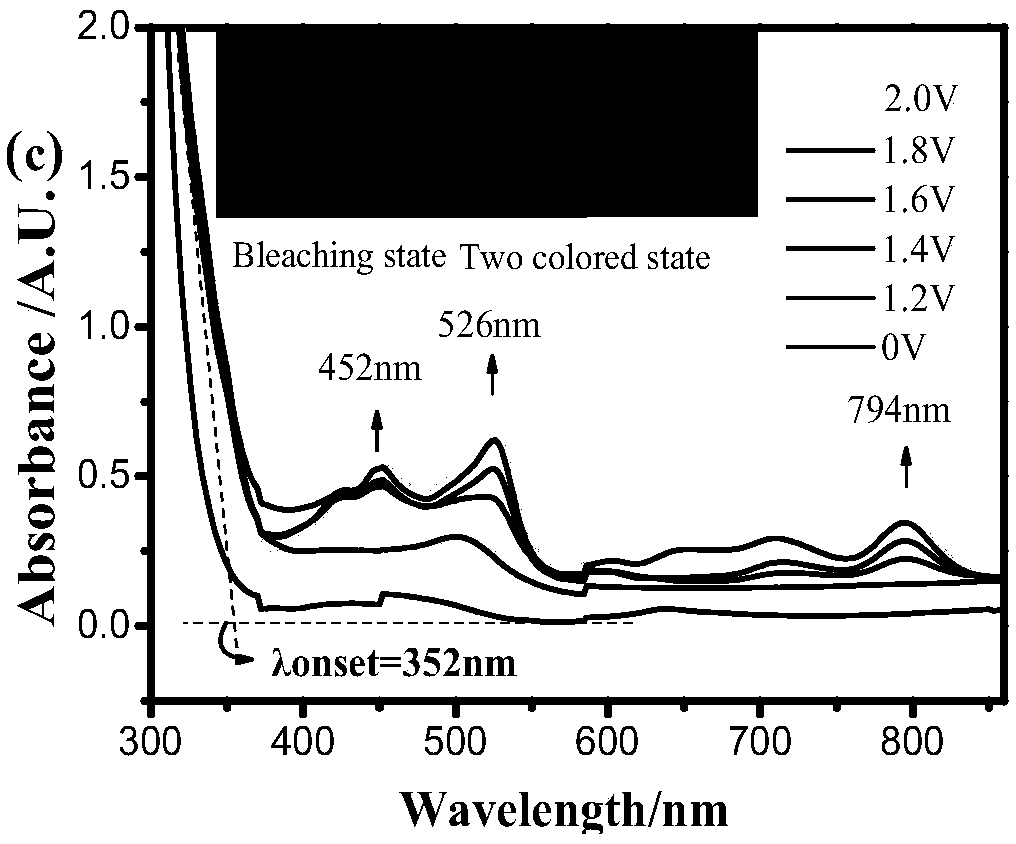
A multicolor electrochromic material based on four-membered ring pyridines with different substituents
A cyclic pyridine, multi-color technology, applied in the application of new electrochromic materials, the field of quaternary ring pyridine multi-color electrochromic materials, can solve the problems of long response time, single color change, etc., and achieve convenient post-processing, Broad application prospects and the effect of expanding the range of color choices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Such as figure 1 As shown, the preparation method of the multi-color changing four-membered ring pyridine derivative provided by the embodiment of the present invention comprises the following steps:
[0036] S101: Add 1,2,3,4-tetra-(4-pyridine)cyclobutane represented by formula II to the organic solvent, and then dropwise add halogenated hydrocarbon represented by formula III, 1,2,3,4-tetra The mol ratio of -(4-pyridine) cyclobutane to halogenated hydrocarbon is 1:4-4.5;
[0037] S102: Then stir the reaction at a constant temperature of 80-90°C. After the reaction is completed, cool to room temperature, add three times the volume of diethyl ether as a precipitant, filter, and dry the filter cake to obtain 1,2,3,4-tetra-( 4-pyridine) cyclobutane tetrapyridinium salt, ie R 1 , R 2 , R 3 and R 4 same and X 1 、X 2 、X 3 and x 4 the same compound of formula I.
[0038] The chemical reaction formula of the preparation method of the four-membered ring pyridine deriva...
preparation Embodiment 1
[0043]
[0044] Add 0.3g (0.82mmol) of 1,2,3,4-tetra-(4-pyridine) cyclobutane into 8ml of dimethylformamide, then slowly drop into 2ml of iodomethane dissolved in 0.58g (4.1mmol) Dimethylformamide, stirred at 80°C for 36 hours, added three times the volume of ether as a precipitant, filtered, washed the filter cake with anhydrous ether, and dried at 60°C to obtain N,N',N",N" '-Tetramethyl-1,2,3,4-tetrakis(4-pyridyl)cyclobutane quaternary ammonium iodide 1 (yellow black solid), yield 86.0%; 1 HNMR (600MHz, DMSO-d 6 ), δ (ppm): 9.02 (d, 8H, J = 6.60Hz), 8.23 (d, 8H, J = 6.60Hz), 4.67 (s, 4H), 4.34 (s, 12H); 13 CNMR (150MHz, DMSO-d 6 ): 156.28, 145.99, 127.19, 48.05, 48.02. The structure of the product is completely consistent with the above structural formula 1 as determined by NMR.
preparation Embodiment 2
[0046]
[0047] Add 0.3g (0.82mmol) of 1,2,3,4-tetra-(4-pyridine) cyclobutane into 8ml of dimethylformamide, then slowly drop into 2ml of 0.79g (4.1mmol) of n-bromo Dimethylformamide of octane, stirred at 80°C for 36 hours, added three times the volume of diethyl ether as a precipitant, filtered, washed the filter cake with anhydrous ether, and dried at 60°C to obtain N,N',N” ,N"'-tetra-n-octyl-1,2,3,4-tetra(4-pyridyl)cyclobutane pyridinium bromide 2 (light brown solid), yield 56.1%; 1 HNMR (600MHz, DMSO-d 6 )δ (ppm) 9.15 (8H, d, J = 66.0), 8.36 (8H, d, J = 66.0), 4.78 (4H, s), 4.59 (8H, t, J = 15), 1.89 (8H, m ,J=6.0),1.24-1.29(40H,m,J=30.6),0.85(12H,t,J=15.2); 13 CNMR (150MHz, DMSO-d 6 ) 156.87, 145.08, 127.71, 60.76, 47.99, 34.85, 31.58, 28.88, 25.93, 22.46, 14.36.
[0048] The structure of the product is completely consistent with the above structural formula 2 as determined by NMR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com