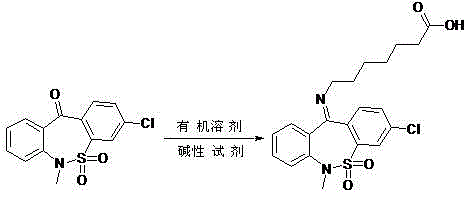
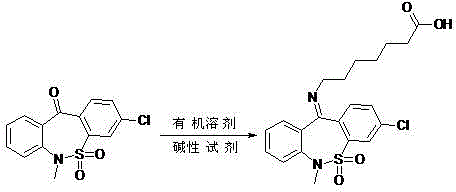
Preparation method of tianeptine sodium impurity D
A technology for tianeptine sodium and impurities, which is applied in the field of preparation of tianeptine sodium impurity D, can solve problems such as no public data report on the synthesis method of impurity D, achieve high purity and solve the problems of quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 30.7g 3-chloro-6-methyldibenzo[c,f][1,2]thiazepine-11(6H)-one S,S-dioxide and 18.2g 7-aminoheptanoate Add salt to 153.5ml of toluene, add 8g of sodium hydroxide, heat and reflux for 5 hours, after the reaction is completed, cool to room temperature, adjust pH=5~6 with 1N dilute hydrochloric acid, filter, wash with a small amount of ethanol, and dry to obtain 36g of crude product.
[0016] ②Add 36g of the crude product to 180ml of ethanol, heat to reflux for 30 minutes, cool to room temperature, filter, and dry to obtain 30.8g of pure impurity D, with a purity greater than 99.5% and a total yield of 71%.
Embodiment 2
[0018] 61.4g3-chloro-6-methyldibenzo[c,f][1,2]thiazepine-11(6H)-one S,S-dioxide and 40g 7-aminoheptanoic acid hydrochloride Add salt to 368.4ml of toluene, add 17.6g of sodium hydroxide, heat and reflux for 6 hours, after the reaction is complete, cool to room temperature, adjust pH=5~6 with 1N dilute hydrochloric acid, filter, wash with a small amount of ethanol, and dry to obtain 73g of crude product.
[0019] ②Add 73g of the crude product to 438ml of ethanol, heat to reflux for 30 minutes, cool to room temperature, filter, and dry to obtain 62.2g of pure impurity D, with a purity greater than 99.5%, and a total yield of 71%.
Embodiment 3
[0021] 200g 3-chloro-6-methyldibenzo[c,f][1,2]thiazepine-11(6H)-one S,S-dioxide and 130g 7-aminoheptanoate hydrochloride Add 1.1L of toluene, add 57g of sodium hydroxide, heat and reflux for 5.5 hours, after the reaction is completed, cool to room temperature, adjust pH=5~6 with 1N dilute hydrochloric acid, filter, wash with a small amount of ethanol, and dry to obtain 238g of crude product.
[0022] ②Add 238g of the crude product to 1.3L of ethanol, heat to reflux for 30 minutes, cool to room temperature, filter, and dry to obtain 203g of pure impurity D, with a purity greater than 99.5%, and a total yield of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com