Preparation method of agomelatine solid preparation
A solid preparation and carrier technology, which is applied in the field of medicine, can solve the problems of reducing production efficiency and cumbersome production, and achieve the effects of simple method, saving cost and production time, and good in vitro dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
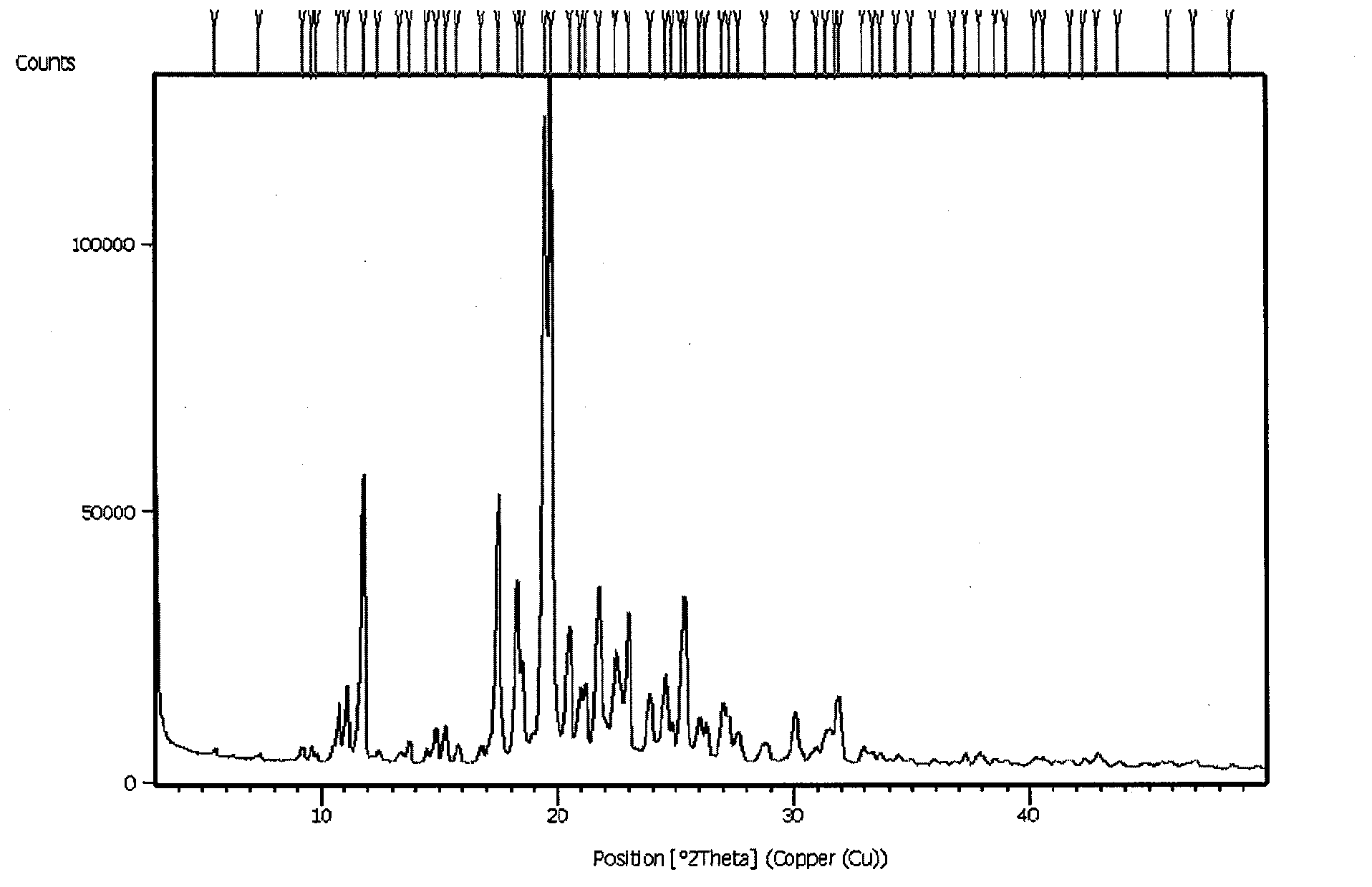
Image
Examples
Embodiment 1
[0023] Preparation of the composition of agomelatine:
[0024] Weigh 10 g of crystal form II agomelatine and 20 g of crospovidone (model Kollidon CL) and mix them evenly, then add them to an oven at 160° C. for 10 minutes, take them out and pass through a sieve to cool.
Embodiment 2
[0026] Preparation of the composition of agomelatine:
[0027] Weigh 100 g of agomelatine of crystal form VI and 300 g of crospovidone (model Kollidon CL-M) and add them to a jacketed granulator for granulation. The temperature is set at 105° C. and stirred for 5 minutes. Take out and sieve to cool.
Embodiment 3
[0029] Preparation of the composition of agomelatine:
[0030] Weigh 10 g of crystal form II agomelatine and 40 g of crospovidone (Polyplasdone XL) and mix them evenly, then add them to an oven at 140° C. for 10 minutes, take them out and pass through a sieve to cool.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com