Simple synthesis method of benzophenanthrene
A triphenylene and simple technology, which is applied in the field of preparation of 9,10-triphenylene, can solve the problems of high requirements, high energy consumption, large production investment, etc., and achieves low investment in reaction equipment, low energy consumption and economical efficiency. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
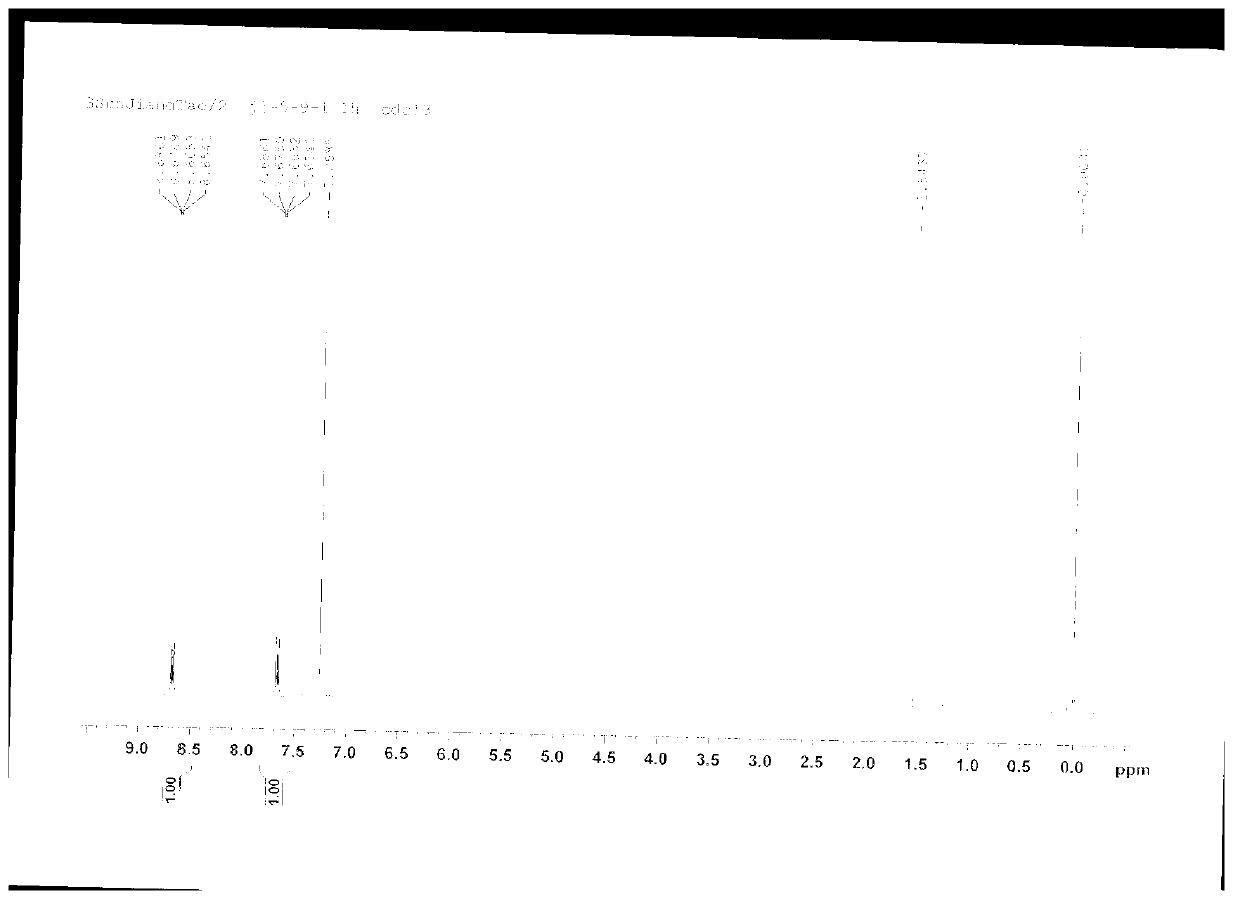
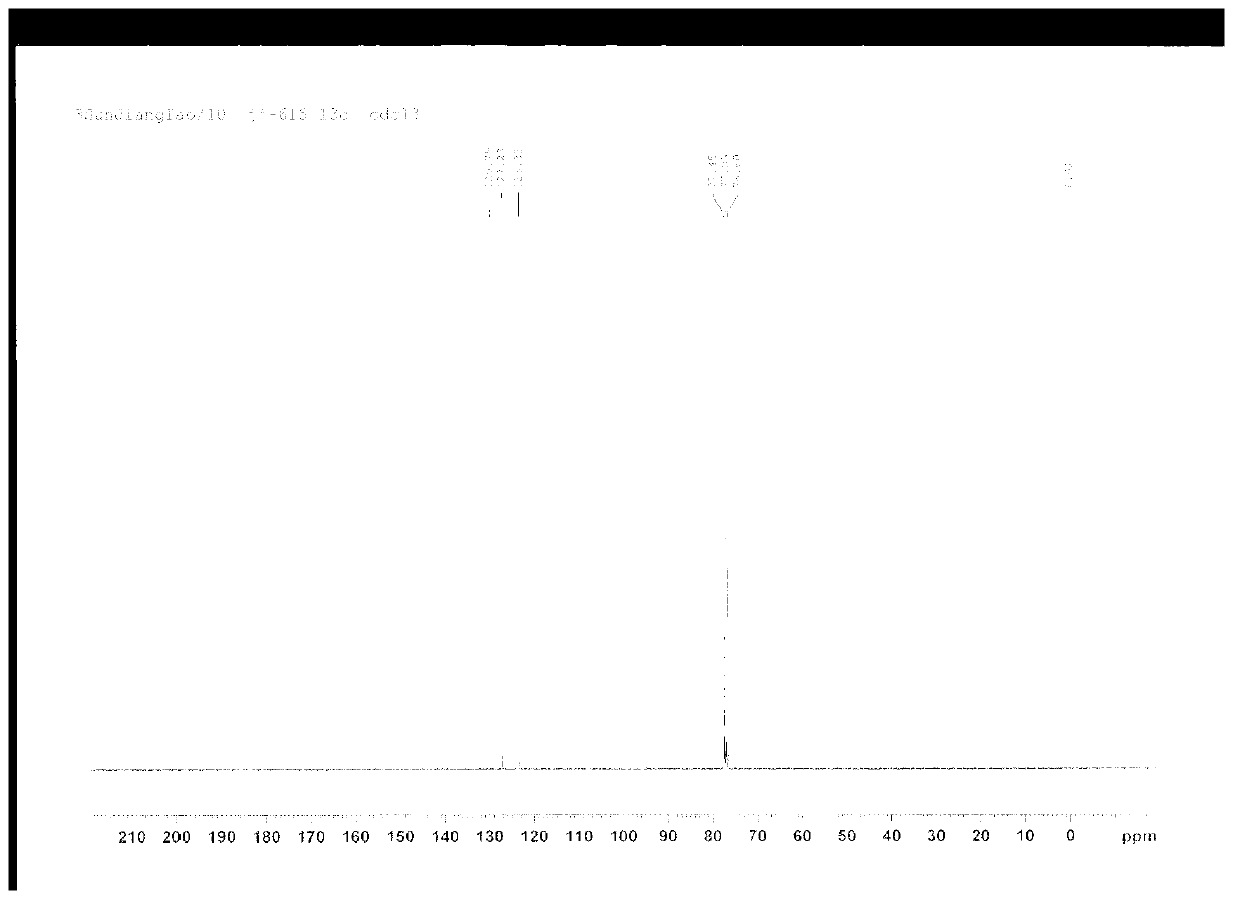
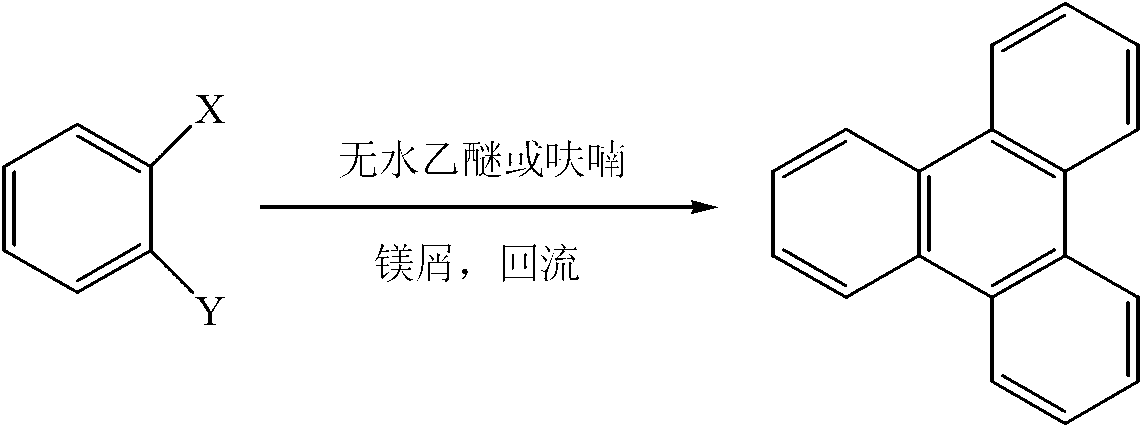
[0019] Add 17.5g (0.72mol) of magnesium chips, 105g (0.6mol) of o-fluorobromobenzene, and 500ml of tetrahydrofuran into a 1L reaction flask equipped with a stirrer, a thermometer, a drying tube, and a reflux condenser, and heat to reflux for 0.5 hours while stirring. , then dropwise add a solution of 110g (0.63mol) o-fluorobromobenzene in 200ml tetrahydrofuran, drop it in 1.5 hours, finish the dropwise, keep warm for 4 hours, cool, filter with suction, distill off the organic phase tetrahydrofuran to obtain a yellow oil, Recrystallized with petroleum ether and ethyl acetate (volume ratio: 5:1) to obtain colorless needle-like crystals, which emit blue fluorescence under ultraviolet irradiation, with a yield of 91%. The carbon spectrum identified it as 9,10-triphenylene. The recovered tetrahydrofuran, petroleum ether and ethyl acetate can be recycled.
Embodiment 2
[0021] In a 1L reaction flask equipped with a stirrer, a thermometer, a drying tube, and a reflux condenser, add 17.5g (0.72mol) of magnesium chips, 105g (0.6mol) of o-fluorobromobenzene, 400ml of anhydrous tetrahydrofuran and 100ml of anhydrous 2 - Methylfuran, heated to reflux under stirring for 0.5 hours, then dropwise added a solution of 110g (0.63mol) o-fluorobromobenzene in 200ml tetrahydrofuran, dripped in 1.5 hours, the dropwise addition was completed, kept the reaction for 5.5 hours, cooled, suction filtered, Evaporate the organic phase tetrahydrofuran and 2-methylfuran to obtain a yellow oil, which is recrystallized from petroleum ether and ethyl acetate (volume ratio: 5:1) to obtain colorless needle-like crystals that radiate blue under ultraviolet light. Fluorescence, the yield was 85%, and it was determined to be 9,10-triphenylene by proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum. The recovered furan, petroleum ether and ...
Embodiment 3
[0023] Add 17.5g (0.72mol) of magnesium chips, 105g (0.6mol) of o-fluorobromobenzene, and 800ml of anhydrous diethyl ether in sequence to a 1L reaction flask equipped with a stirrer, a thermometer, a drying tube, and a reflux condenser, and heat to reflux under stirring. 0.5 hours, then dropwise add the solution of 110g (0.63mol) o-fluorobromobenzene in 50ml of anhydrous ether, drop it in 1.5 hours, end the dropwise addition, keep warm for 12 hours, cool, filter with suction, distill off the organic phase ether to get The yellow oil was recrystallized with petroleum ether and ethyl acetate (volume ratio: 5:1) to obtain colorless needle-like crystals, which emitted blue fluorescence under ultraviolet irradiation, and the yield was 87%. , and C-NMR spectrum identified as 9,10-triphenylene. The recovered ether, petroleum ether and ethyl acetate can be recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com