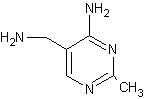
Synthetic method of vitamin B1 intermediate
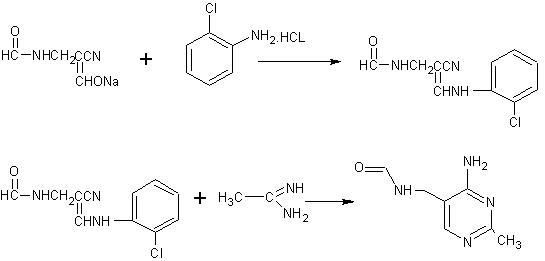
A synthesis method and intermediate technology, which are applied in the field of chemical drug preparation, can solve the problems of large dust, high labor intensity, high energy consumption in the recovery process of o-chloroaniline, etc., and achieve the effect of optimizing technological process and reducing labor intensity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The present invention is illustrated with examples below, and these examples are intended to help understand technical means of the present invention. However, it should be understood that these embodiments are only exemplary, and the present invention is not limited thereto.
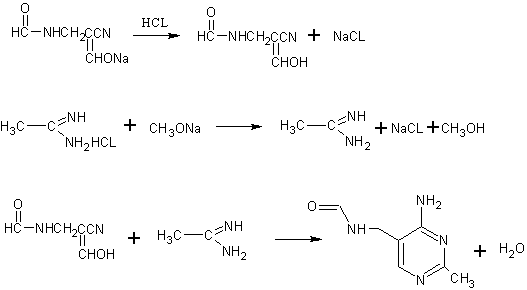
[0019] 1. Preparation of compound α-enolyl-β-formamidopropionitrile (Ⅲ)
[0020] Disperse 68g (0.32mol, content 70%) of α-sodium formyl-β-formamidopropionitrile (IV) in 100g of methanol in a four-neck flask, feed 0.4mol of dry hydrochloric acid gas, and keep the temperature at At about 20°C, react for 1 hour and then filter, the filtrate is 138g of methanol solution of α-enolyl-β-formamidopropionitrile (Ⅲ).
[0021] 2. Preparation of compound acetamidine (Ⅱ)
[0022] Put 50g of acetamidine hydrochloride (0.48mol, content ≥ 91.0%) into a dry three-necked flask, keep warm in a water bath at about 25°C, add 95g of liquid sodium methoxide (0.49mol, content 28%) dropwise with a constant pressure fun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com