Fluorine-containing phenyl hydrogen-containing siloxane monomer and preparation method thereof
A technology of fluorophenyldichlorosilane and fluorophenyldialkoxysilane, which is applied in the field of fluorine-containing phenyl hydrogen-containing siloxane monomer and its preparation, and can solve the problems of non-oil resistance and non-containing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
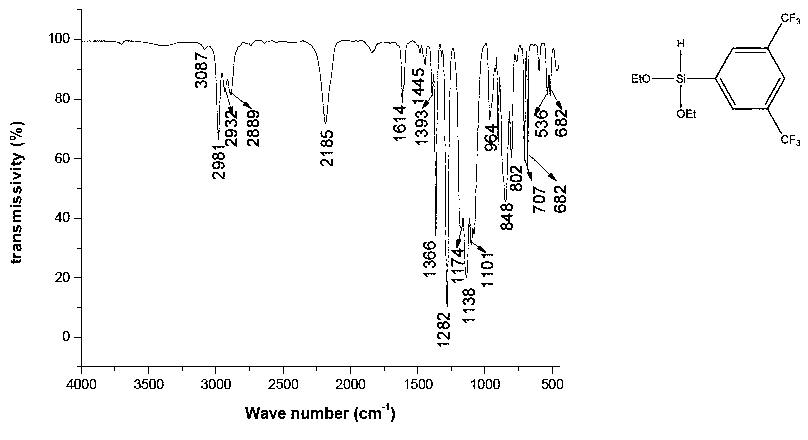
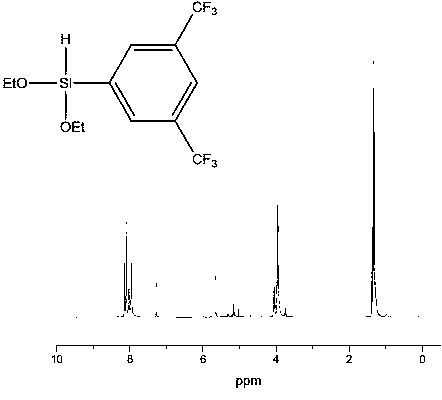
[0019] Example 1 Synthesis of 3,5-bis(trifluoromethyl)phenyldiethoxysilane
[0020]
[0021] Add 5.00 g of magnesium chips and ether into a four-necked flask equipped with a spherical condenser, a thermometer, and a constant pressure dropping funnel. in N 2 Under protection, stir, heat to 35°C, slowly add 58.60g of a mixture of 3,5-bis(trifluoromethyl)bromobenzene and ether dropwise, and keep the reaction temperature at about 35°C. After the dropwise addition, reflux and stir at 35°C for 4 hours to obtain 3,5-bis(trifluoromethyl)bromophenyl Grignard reagent.
[0022] Add 31.42g diethoxychlorosilane in the four-necked flask equipped with spherical condenser, stirrer, thermometer and constant pressure dropping funnel, 2 Under protection, slowly add the cooled 3,5-bis(trifluoromethyl)bromophenyl Grignard reagent according to the substance ratio of 1:1, continue the reaction for 1 hour after the addition, and raise the temperature to 60°C for 2 Hour. Suction filtration afte...
example 2
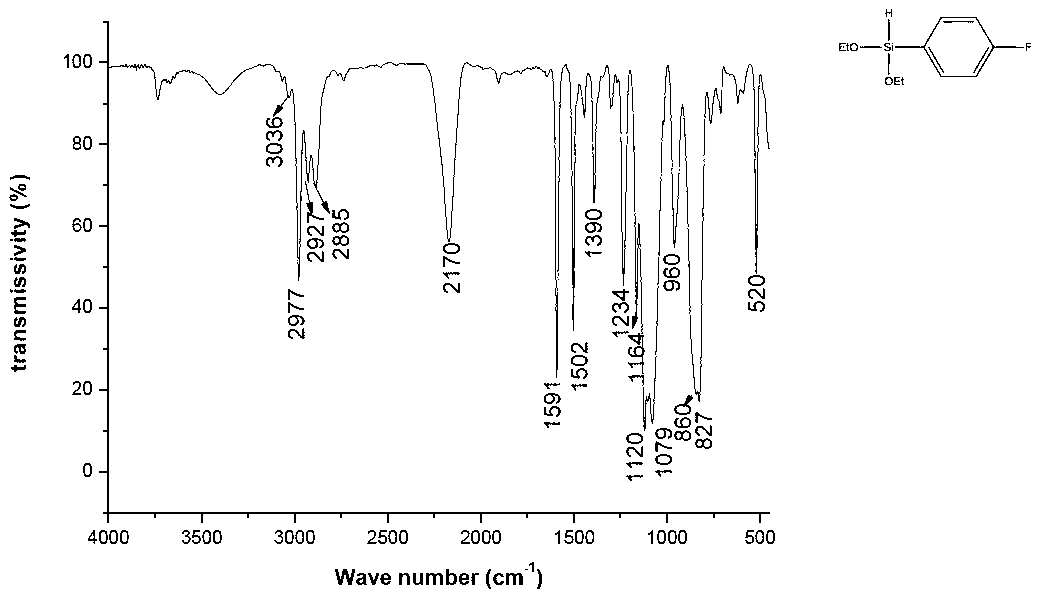
[0025] The synthesis of example 2 p-fluorophenyldiethoxysilane
[0026]
[0027] Add 5.00 g of magnesium chips and ether into a four-neck flask equipped with a spherical condenser, a thermometer, and a constant pressure dropping funnel. in N 2 Stir under protection, heat to 35°C, and slowly add 35.00g of a mixture of p-fluorobromobenzene and ether dropwise according to the ratio of the substance to 1:1, and keep the reaction temperature at about 35°C. Reflux and stir at 35°C for 4 hours to obtain p-fluorobromophenyl Grignard reagent.
[0028] Add 31.00g diethoxychlorosilane in a four-necked flask equipped with a spherical condenser, stirrer, thermometer and constant pressure dropping funnel, 2 Under protection, the cooled p-fluorobromophenyl Grignard reagent was slowly added, and the reaction was continued for one hour after the dropwise addition, and then the temperature was raised to 60° C. for 2 hours. Suction filtration after the reaction was completed, and the filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com