Novel febuxostat pharmaceutical co-crystal and preparation method thereof
A technology of febuxostat and medicine, applied in the field of novel febuxostat co-crystal and its preparation, to achieve the effect of improving stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
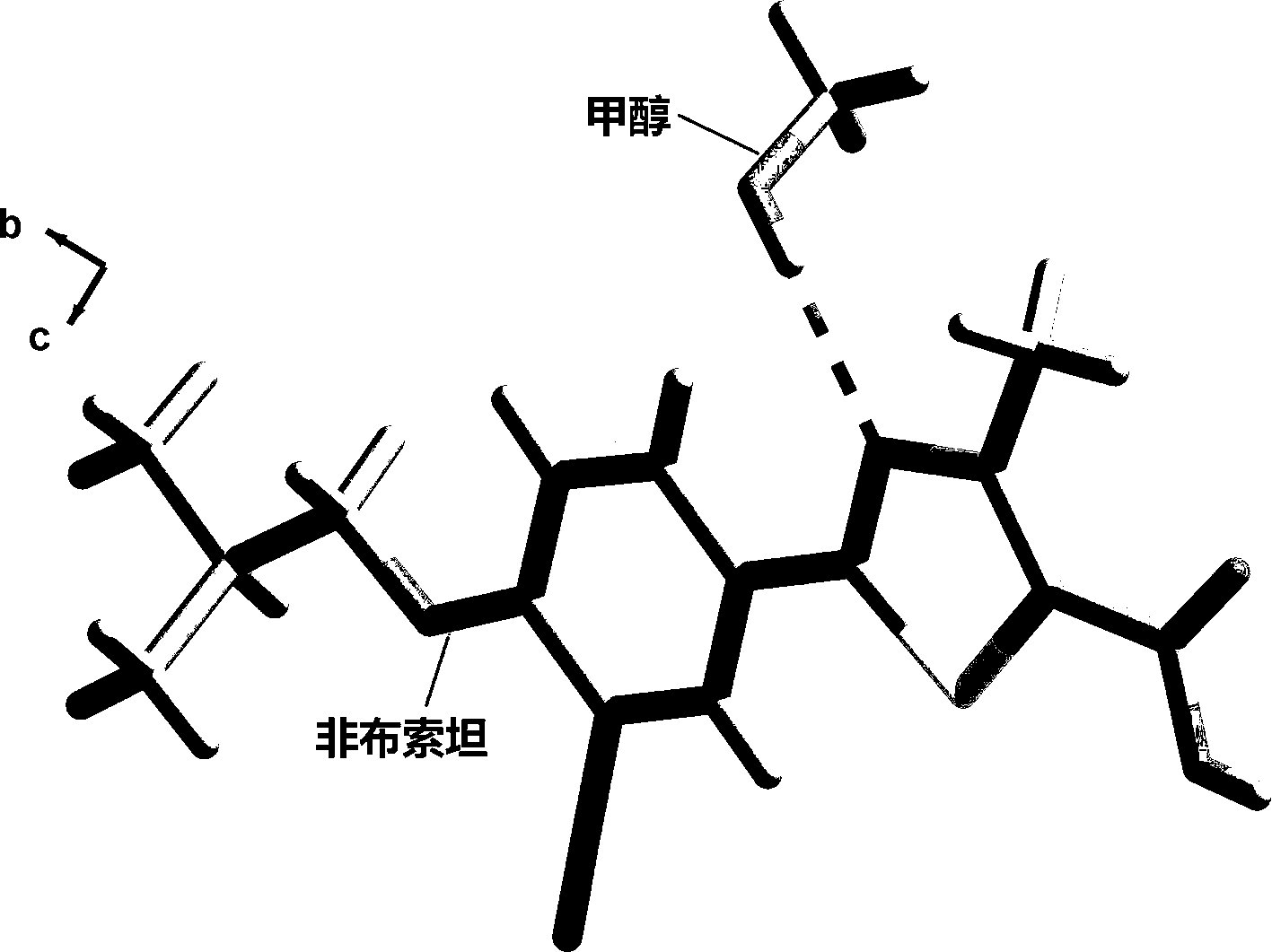
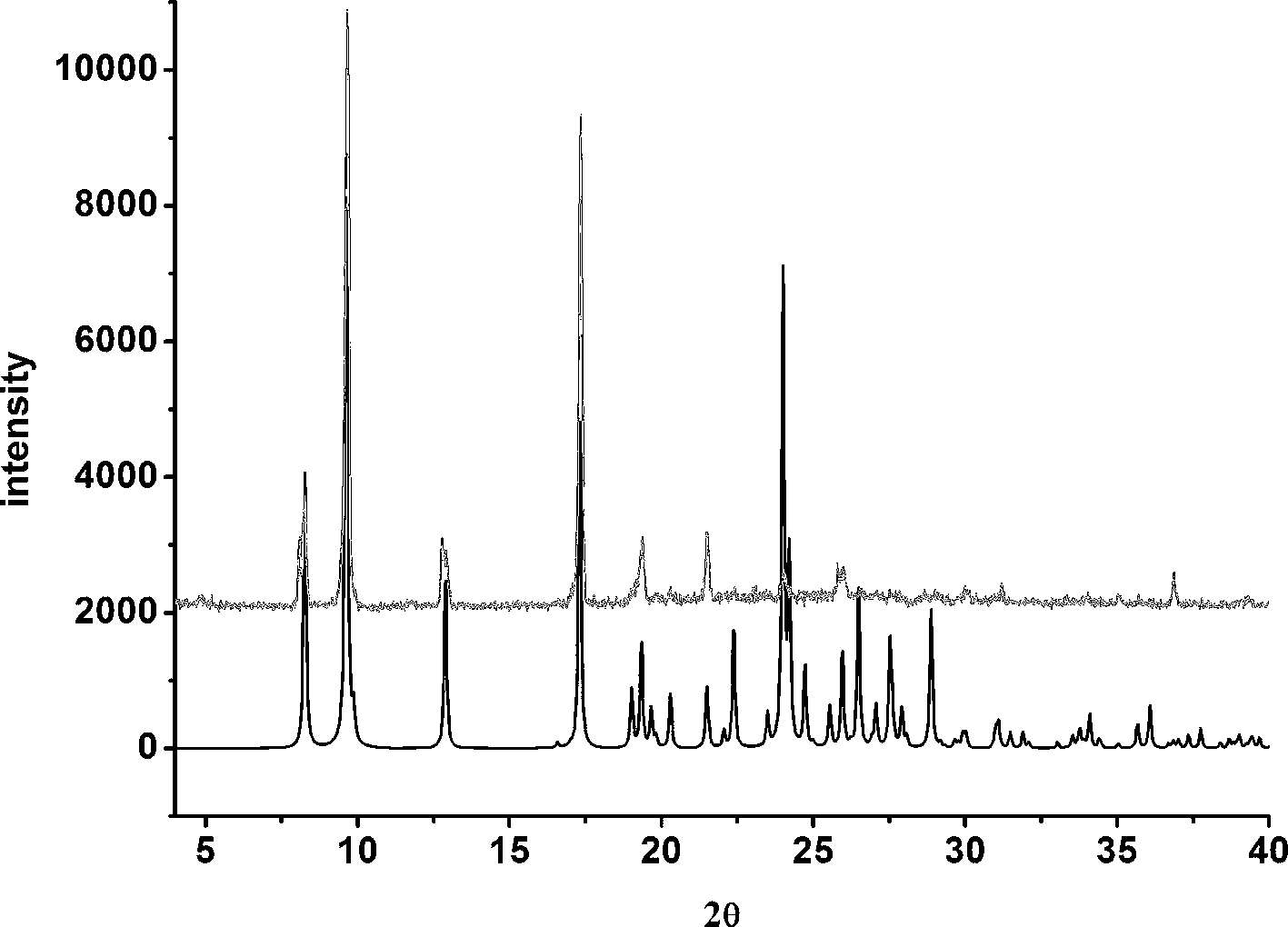
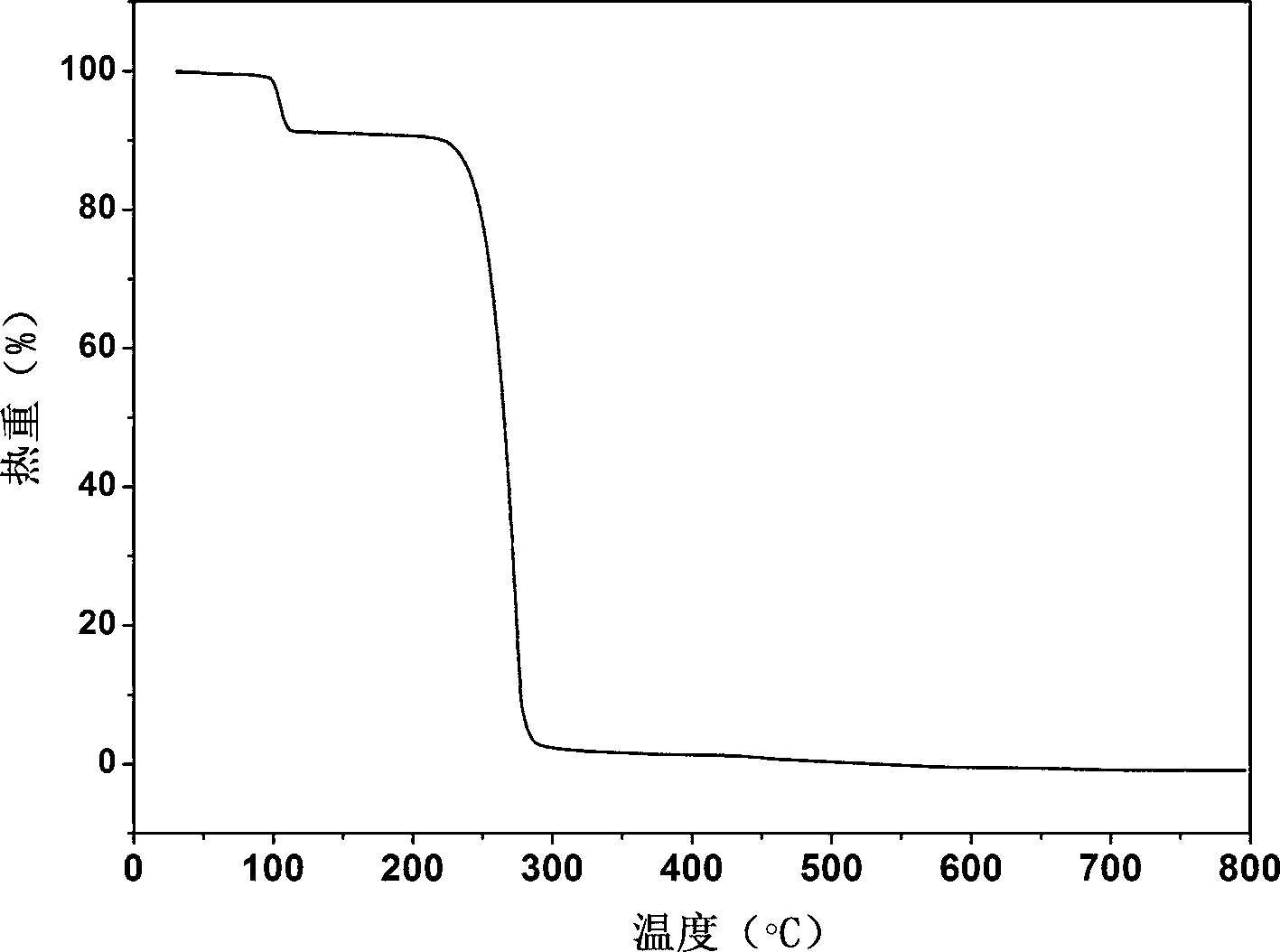
[0025] Example 1: Synthesis of co-crystals using febuxostat and methanol:
[0026] Weighing: The reactant is fed according to 20.00 mg febuxostat. Accurately weigh 20.00 mg febuxostat with an analytical balance.
[0027] Dissolution of the raw material drug: Use a 5ml pipette to accurately measure 2ml of dichloromethane into a 20ml transparent glass vial container, and stir for 1 hour to dissolve almost all the solids. Then 3ml of methanol was added until all the solids were dissolved and the solution turned into a brownish-yellow clear liquid.
[0028] Solvent room temperature volatilization heating method: After the solid is completely dissolved, stir in a closed environment for 2 hours, then take out the stirring bar, seal the bottle mouth with tin foil, prick a few small holes with a needle, and let it stand for volatilization. After about 6 days, light yellow transparent block crystals precipitated in the bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com