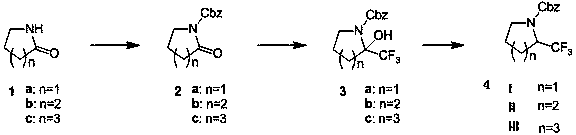
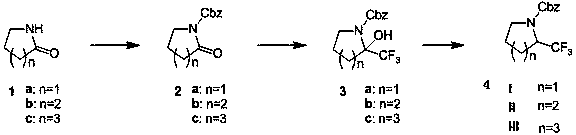
The preparation method of 2-trifluoromethyl-1-benzyloxycarbonyl-1-azacycloalkane
A technology of azacycloalkane and benzyloxycarbonyl, which is applied in the field of preparation of 2-trifluoromethyl-1-benzyloxycarbonyl-1-azacycloalkane, can solve the problem that the α-substituted azacycloalkane has long steps, Low yield, unsuitable for amplification, etc., to achieve the effects of low yield, improved yield, and improved drug-like properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of 2-oxa-benzyloxycarbonyl cycloheptylamine 2c
[0019]
[0020] Steps:
[0021] Under nitrogen protection, compound cycloheptamide 1c (20 grams, 0.23 moles) and tetrahydrofuran (200 milliliters) were added in a dry three-necked flask, and n-butyllithium (94 milliliters, 0.23 moles, 2.5 M n-hexane solution). The reaction solution was stirred at -78°C for 60 minutes, and then a solution of benzyl chloroformate (40 g, 0.23 mol) in THF (50 mL) was slowly added. Slowly rise to room temperature and stir for 10 hours, quench with saturated ammonium chloride aqueous solution, extract with ethyl acetate, dry and concentrate the organic phase to obtain 53 g of product 2-oxa-benzyloxycarbonylcycloheptylamine 2c, yield 100%. used directly in the next reaction.
Embodiment 2
[0022] Example 2: Preparation of 2-oxa-1-benzyloxycarbonylpyrroline 2a
[0023] When n=1, the operation is the same as in Example 1. The yield is 100%. used directly in the next reaction.
Embodiment 3
[0024] Example 3: Preparation of 2-oxa-1-benzyloxycarbonylpiperidine 2b
[0025] When n=2, the operation is the same as in Example 1. The yield is 70%. used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com