Preparation process of polyhydroxy isoflavone
A technology of hydroxy isoflavone and trihydroxy isoflavone, which is applied in the field of preparation technology of polyhydroxy isoflavone, can solve the problem that there is no economical and efficient laboratory research synthesis technology, no mass production preparation technology report, and inability to realize mass production preparation and other problems, to achieve the effects of high yield and purity, simple and efficient carbon-enhancing and cyclization process, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
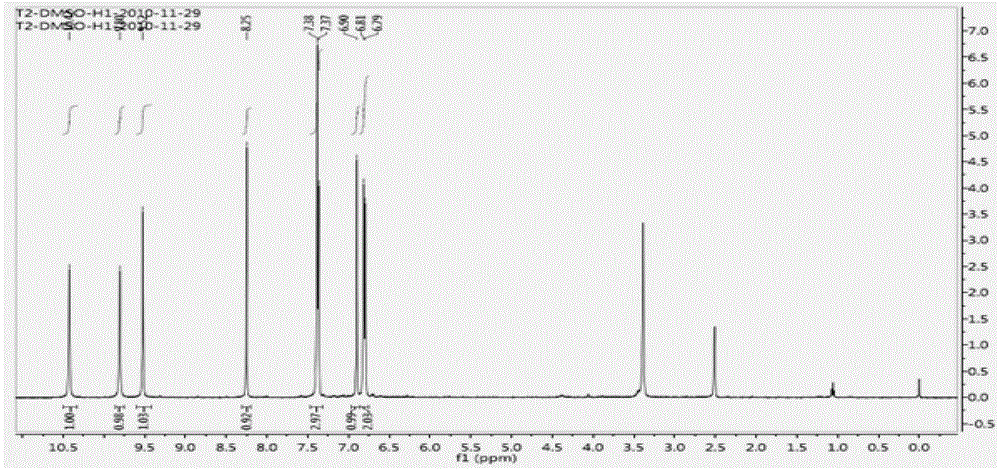
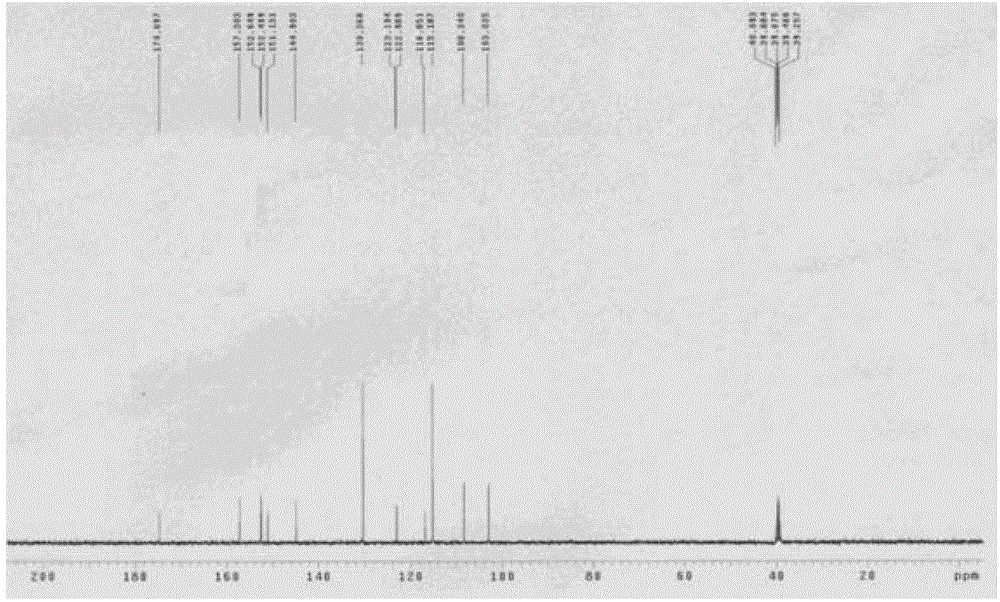
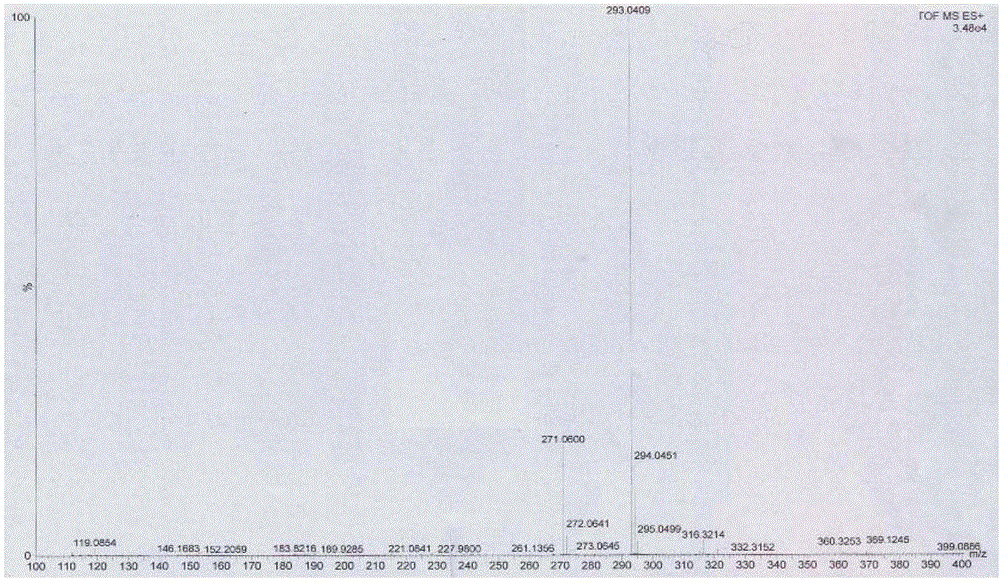
[0041] Embodiment 14', the preparation of 6,7-trihydroxyisoflavones (T2)
[0042]
[0043] 1. Preparation of 2,4`-dihydroxy-4,5-dimethoxydeoxybenzoin (compound 3)
[0044] 2kgZnCl 2 Add it into 6L of 1,4-dioxane solution, stir and disperse it, and feed 1.6 kg of HCl gas within 6 hours, then stop the feeding of HCl gas, and add 2 kg of p-hydroxybenzonitrile (compound 1) in batches under vigorous stirring, Add 2kg of 3,4-dimethoxyphenol (compound 2) after 6 hours of reaction, stop stirring after 36 hours at room temperature, add the reaction solution to 100L 5% HCl solution at 85°C for 2 hours and precipitate a large amount of light yellow precipitate, filter and wash with hot water Precipitate to neutrality, dry and weigh the product—2,4`-dihydroxy-4,5-dimethoxydeoxybenzoin (compound 3) 3.175kg, yield 85%, purity 96% (HPLC); melting point (mp): 174~175.5℃; 1 H NMR (400MHz, DMSO) δppm: 12.50 (1H, s, 2-OH), 9.32 (1H, s, 4′-OH), 7.42 (1H, s, 6-H), 7.09, 7.11 (2H, d ,J=8.6Hz...
Embodiment 23
[0049] Embodiment 23', 4', the preparation of 6,7-tetrahydroxyisoflavone (T3)
[0050]
[0051] 1. Preparation of 2-hydroxy-3`,4`,4,5-tetramethoxydeoxybenzoin (compound 6)
[0052] 1kg ZnCl 2 Add it into 3.5L of 1,4-dioxane solution, stir and disperse, and feed 0.7kg of HCl gas within 6h, then stop feeding of HCl gas, and pour 1.15kg of 3,4-dimethoxyphenylacetonitrile (compound 5) Add it in batches, add 1 kg of 3,4-dimethoxyphenol (compound 2) in batches after 6 hours of reaction, stop stirring after 36 hours at room temperature, add the reaction solution to 50L 5% HCl solution at 85°C for 2 hours for hydrolysis A large number of light yellow precipitates were precipitated, filtered and washed with hot water until neutral, dried and weighed to obtain the product 2-hydroxy-3`,4`,4,5-tetramethoxydeoxybenzoin (compound 6) 1.768kg, yield 82 %, purity 97% (HPLC), melting point 136.9-137.7°C, 1 H-NMR(DMSO,400MHz)δ(ppm):3.682-3.711(d,6H,J=11.6Hz,O-CH3),3.761(s,3H,O-CH3),3.813(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com