Polysulfone with sulfonated lateral chain and preparation method thereof
A sulfonation, side chain technology, applied in fuel cell parts, battery pack parts, electrical components, etc., can solve the problems of complex purification of polymer products and harsh polymerization conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
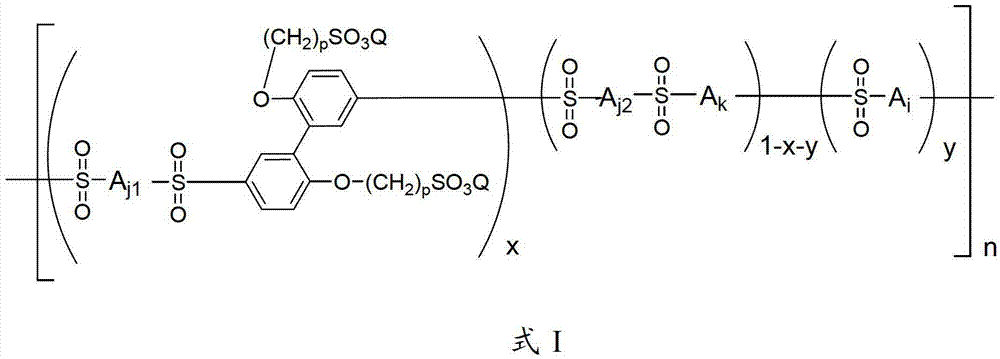
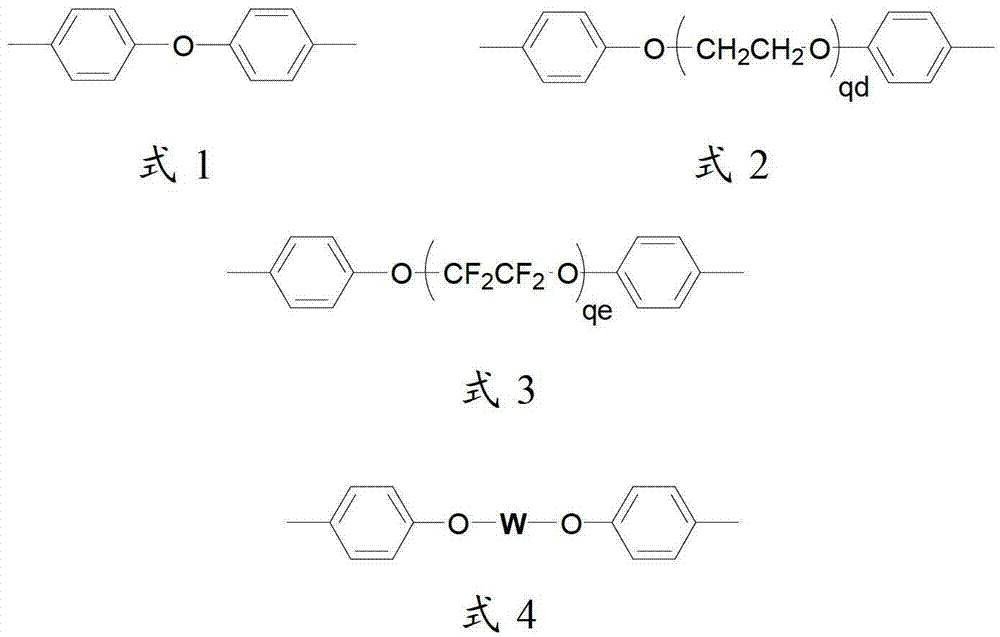
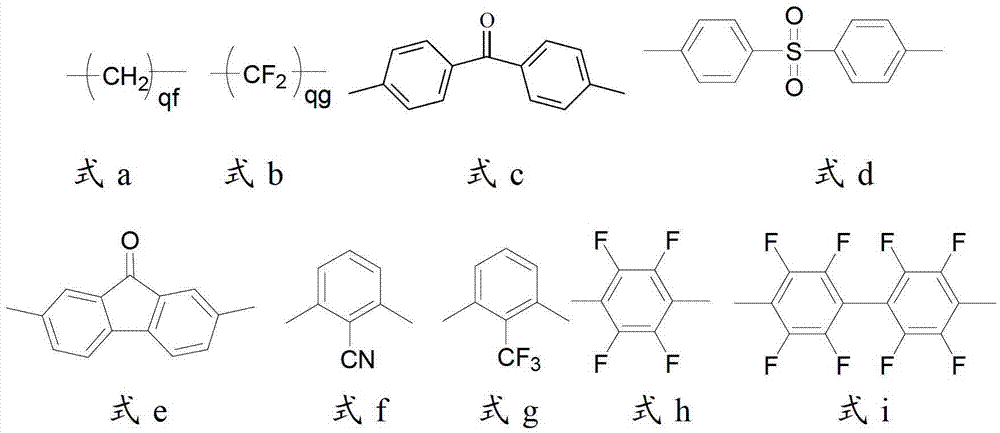
[0052] 0.01 mole of 2,2`-bis(3-sulfonated propoxy)biphenyl disodium, 1.00 mole of 4,4`-diphenyl ether disulfonic acid, 0.99 mole of 2,2`-dimethyl Add oxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction bottle, stir and react at 80°C for 48 hours, pour the reaction liquid into a large amount of deionized water while hot to obtain a polymer precipitate, filter and wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then wash repeatedly with deionized water until the washing solution is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-based polymerization reaction; H NMR spectrum confirms that the stru...
Embodiment 2
[0057] 0.01 mole of 2,2'-bis(3-sulfonated propoxy)biphenyl disodium was synthesized according to the method of Example 1, 1.00 mole of 4,4'-diphenyl ether disulfonic acid, 0.99 mole Add 2,2`-dimethoxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction flask, stir and react at 120°C for 24 hours, pour the reaction solution into a large amount of deionized water while it is hot to obtain a polymer precipitate, filter And wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then repeatedly wash with deionized water until the washing liquid is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-based polymeriz...
Embodiment 3
[0059] In turn, 0.01 moles of 2,2`-bis(3-sulfonated propoxy)biphenyl disodium were synthesized according to the method of Example 1, 1.00 moles of 4,4`-diphenyl ether disulfonic acid, 0.99 moles Add 2,2,-dimethoxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction bottle, stir and react at 130°C for 10 hours, pour the reaction solution into a large amount of deionized water while it is hot to obtain a polymer precipitate, filter And wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then repeatedly wash with deionized water until the washing liquid is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com