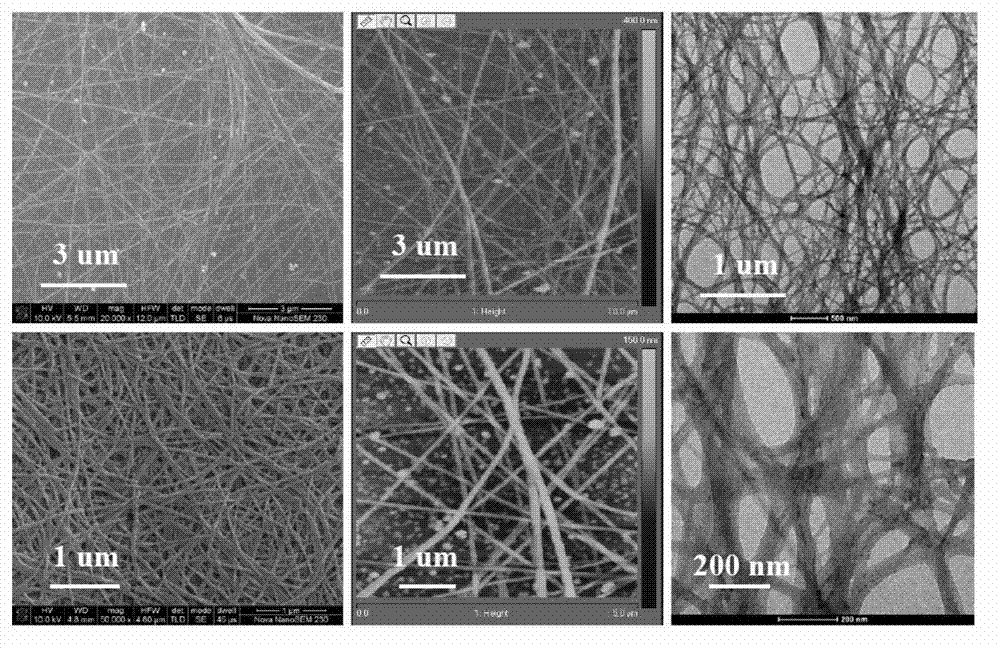
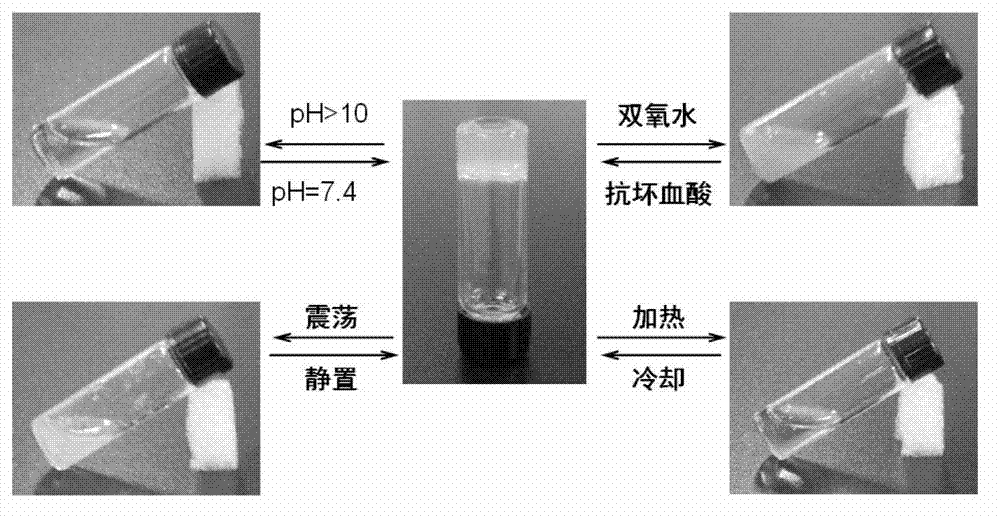
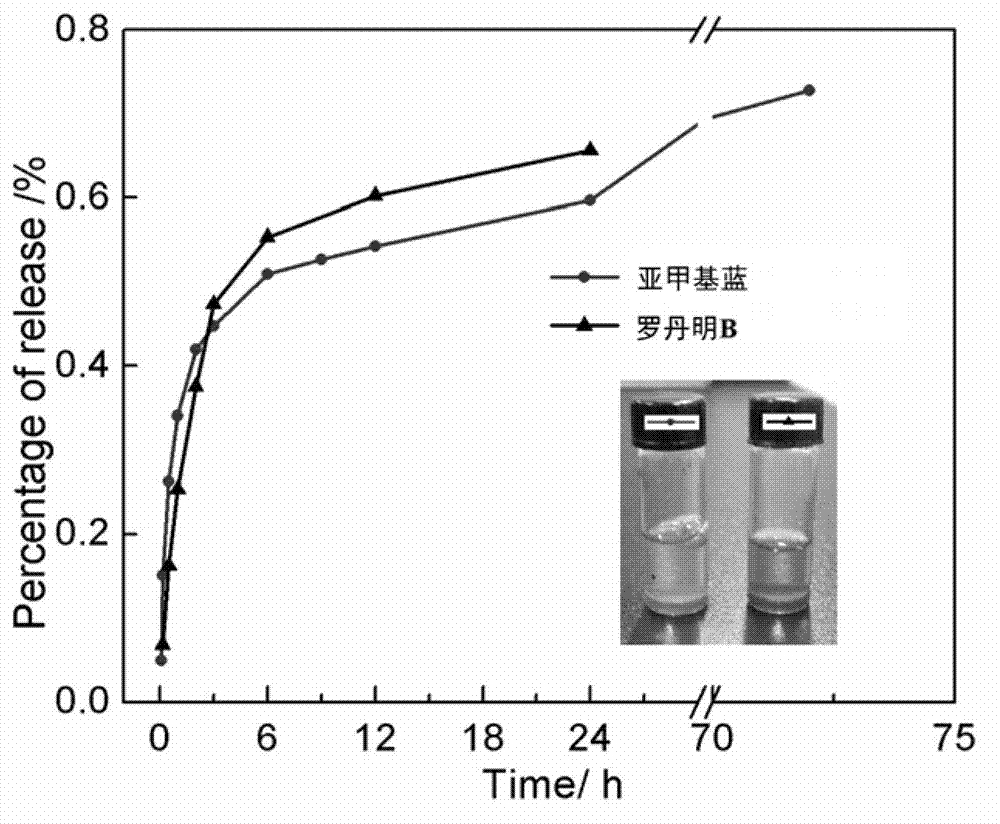
Multi-response supramolecular hydrogel factor, hydrogel and preparation method thereof
A supramolecular hydrogel and hydrogel technology, applied in chemical instruments and methods, medical preparations of non-active ingredients, pharmaceutical formulations, etc., can solve problems such as limited applications, and achieve good anti-tumor and anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh 1.84 g of Fc-COOH (8mM), dissolve it in 250ml of dry dichloromethane, add 1.20g of HOBt and 3.36g of HBTU (8.8mM each) under ice bath. Slowly add about 6 mL of triethylamine dropwise. The reaction was stirred under ice bath for 40 minutes to 1 hour. TLC dot plate to track the reaction. After the reaction is complete, use saturated NaCO sequentially 3 , 5% HCl and distilled water each extract once. The extract was concentrated, purified by column chromatography, and dried in vacuo. Obtained 2.60 g of dark red compound (1).
[0052] Weigh 1.6 g of compound (1), dissolve it in 50 mL of anhydrous dichloromethane, and add 0.86 g of H-Phe-OMe·HCl. Then 2 mL of triethylamine was added dropwise, and reacted for about 10 hours at room temperature. The TLC dot plate tracks the reaction. After the reaction is complete, use saturated NaCO sequentially 3 , 5% HCl and distilled water each extract once. The extract was concentrated, purified by column chromatography, and dri...
Embodiment 2
[0056] Dissolve Fc-COOH, HOBt and HBTU with a molar ratio of 1:1.2:1.2 in anhydrous dichloromethane, and slowly add triethylamine dropwise to pH 8.0-9.0 under ice bath. The reaction is stirred for 40 minutes to 1 hour. TLC dot plate to track the reaction. After the reaction is complete, use saturated NaCO sequentially 3 , 5% HCl and distilled water each extract once. The extract was concentrated, purified by column chromatography, and dried in vacuo. Obtain dark red compound (1).
[0057] The compound (1) and H-Phe-OMe·HCl with a molar ratio of 1:1.2 were dissolved in anhydrous dichloromethane, and then triethylamine was added dropwise to pH 8.0-9.0, and the reaction was stirred at room temperature for about 10 hours. The TLC dot plate tracks the reaction. After the reaction is complete, use saturated NaCO sequentially 3 , 5% HCl and water are extracted once. The extract was concentrated, purified by column chromatography, and dried in vacuo. The yellow compound (2) is obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com