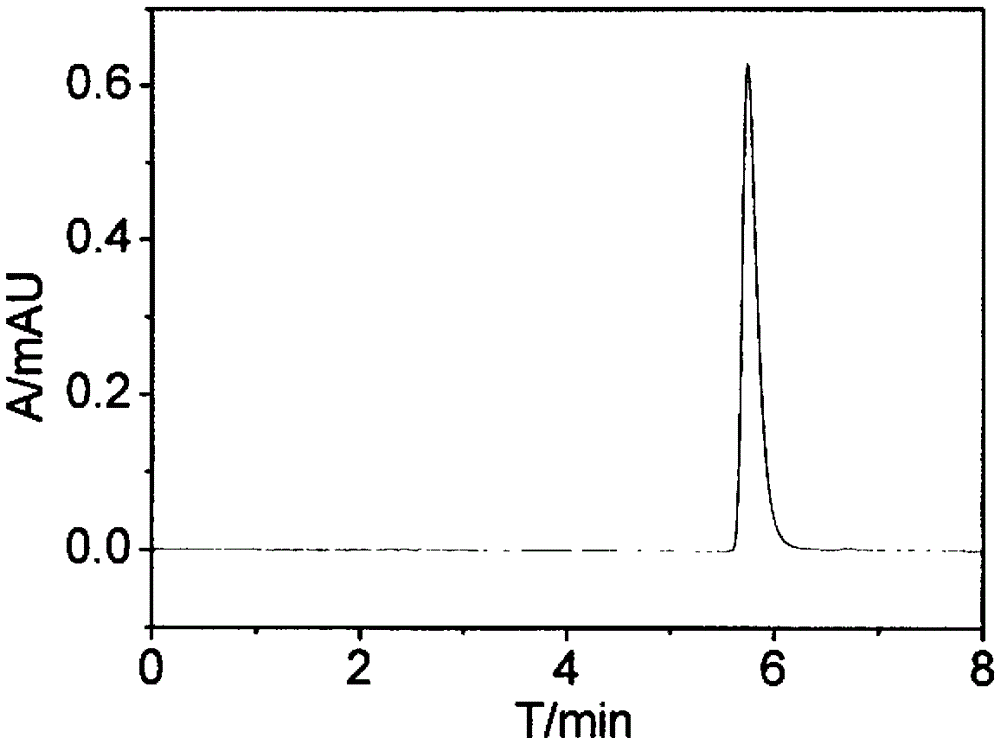
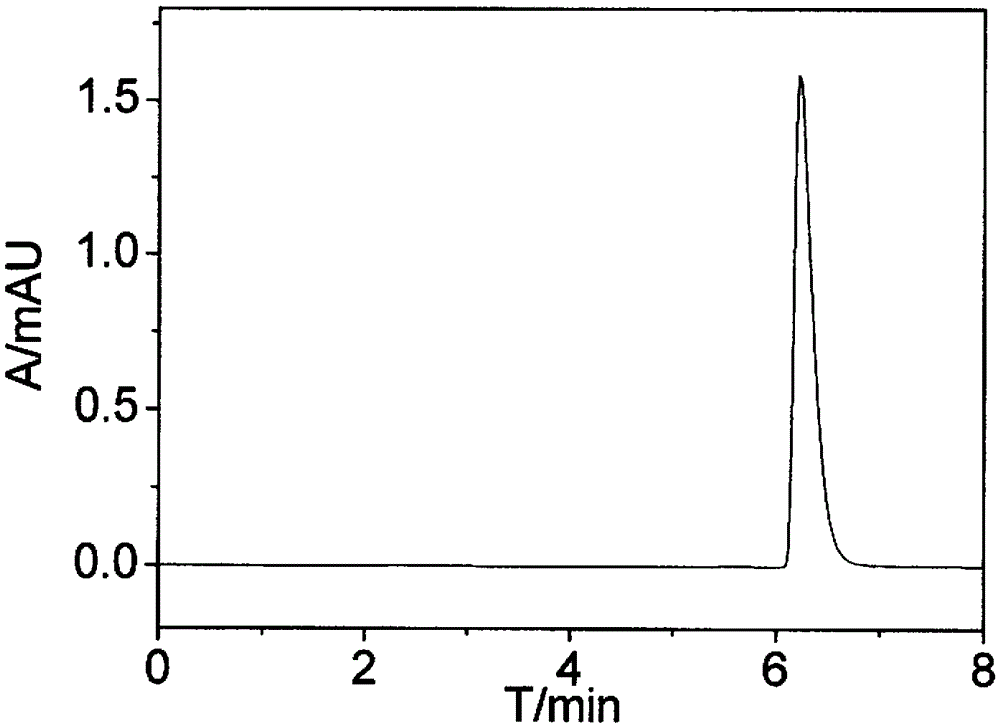
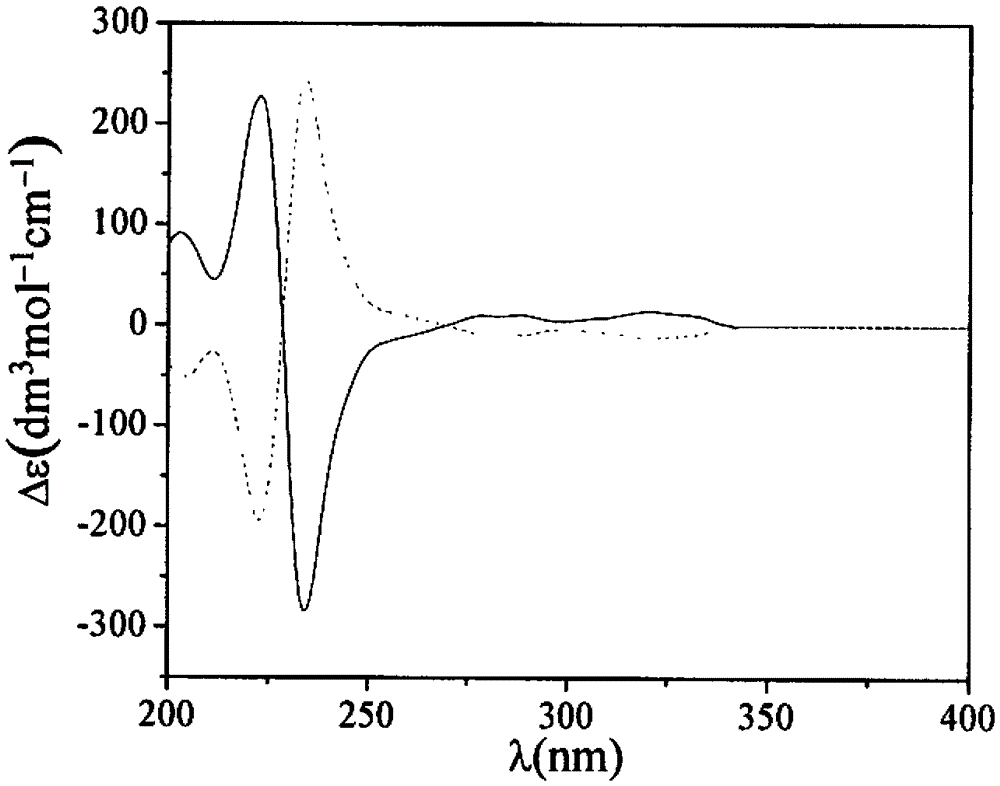
Preferential Crystallization Resolution of Racemic 1,1'-Bi-2-Naphthol
A technology with preferential crystallization and racemization, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Dissolve 0.5000g each of R-BINOL and S-BINOL in 10mL methanol, add 0.1930g Me 4 NCl, heated and stirred to make Me 4 After the NCl is completely dissolved, continue to heat to boiling, and stand for crystallization for 48 hours to obtain 0.6027g and 0.6122g of R-BINOL Me 4 NCl and S-BINOL·Me 4 NCl seeds with yields of 86.97% and 88.34%.
[0017] Combine 5.70g rac-BINOL and 2.20g Me 4 Add NCl to 70mL methanol, heat and stir until racemic BINOL and Me 4 After NCl is completely dissolved, continue heating to boiling, filter while hot, add 3.0mg R-BINOL·Me to the filtrate after cooling to room temperature 4 NCl seed crystal, stirred and crystallized for 16h to obtain the product R-BINOL·Me 4 NCl 0.83g; added racemic BINOL·Me 4 NCl 0.83g and add methanol to a total volume of 70mL, heat and stir until racemization of BINOL and Me 4 After NCl is completely dissolved, continue heating to boiling, add 3.0mg S-BINOL·Me to the filtrate after cooling to room temperature 4 N...
Embodiment 2
[0020] Dissolve 1.0000g each of R-BINOL and S-BINOL in 20mL methanol, add 0.3860g Me 4 NCl, heated and stirred to make Me 4 After the NCl is completely dissolved, continue to heat to boiling, and stand for crystallization for 48 hours to obtain 1.2014g and 1.2490g of R-BINOL Me 4 NCl and S-BINOL·Me 4 NCl seeds with yields of 86.68% and 90.12%.
[0021] 11.40g racemic BINOL and 4.40g Me 4 Add NCl to 140mL methanol, heat and stir until racemic BINOL and Me 4 After NCl is completely dissolved, continue heating to boiling, filter while hot, add 6.0mg R-BINOL·Me to the filtrate after cooling to room temperature 4 NCl seed crystal, stirred and crystallized for 16h to obtain the product R-BINOL·Me 4 NCl 1.76g; supplemented with rac BINOL·Me 4 NCl 1.76g and add methanol to a total volume of 140mL, heat and stir to make racemization of BINOL and Me 4 After NCl is completely dissolved, continue heating to boiling, add 6.0mg S-BINOL·Me to the filtrate after cooling to room tempera...
Embodiment 3
[0024] Dissolve 1.5000g each of R-BINOL and S-BINOL in 30mL methanol, add 0.5790g Me 4 NCl, heated and stirred to make Me 4 After the NCl is completely dissolved, continue to heat to boiling, and stand for crystallization for 48h to obtain 1.8680g and 1.9012g of R-BINOL Me 4 NCl and S-BINOL·Me 4 NCl seeds with yields of 89.85% and 91.45%.
[0025] 17.10g racemic BINOL and 6.60g Me 4 Add NCl to 210mL methanol, heat and stir until racemic BINOL and Me 4 After NCl is completely dissolved, continue to heat to boiling, filter while hot, add 9.0mg R-BINOL·Me to the filtrate after cooling to room temperature 4 NCl seed crystal, stirred and crystallized for 16h to obtain the product R-BINOL·Me 4 NCl 2.01g; added racemic BINOL·Me 4 NCl 2.01g and add methanol to a total volume of 210mL, heat and stir to make racemization of BINOL and Me 4 After NCl is completely dissolved, continue heating to boiling, add 9.0mg S-BINOL·Me to the filtrate after cooling to room temperature 4 NCl s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com