Time-resolved fluorescence comprehensive detection kit of uterine cancer and application thereof
A time-resolved fluorescence and comprehensive detection technology, which is applied in the direction of fluorescence/phosphorescence, measuring devices, and analytical materials, can solve the problems of large differences in judgment results, errors in detection results, and numerous detection steps, achieving fewer steps, shorter time, The effect of improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
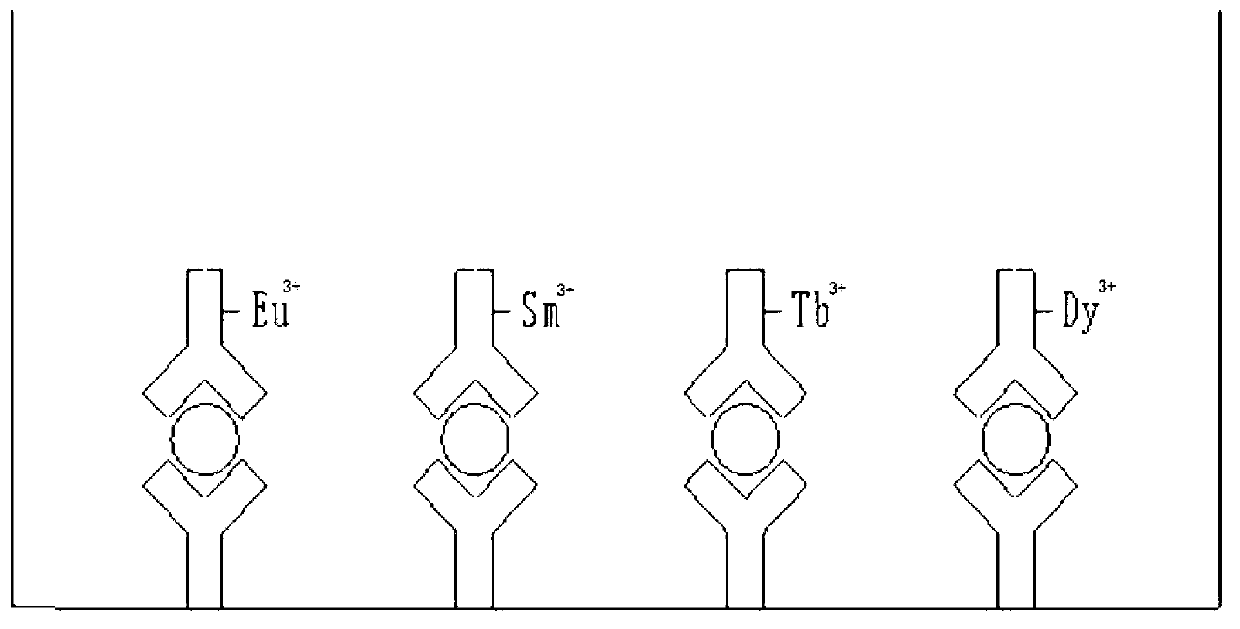
[0033] The time-resolved fluorescence method comprehensive detection kit for uterine cancer provided in this example includes: anti-SCC antibody H00006317-M01, anti-CA125 antibody M32112M, anti-CEA antibody MAM02-008, anti-β-HCG antibody 10R-75154 Antibody-coated microplates composed of a mixture of monoclonal antibodies; with Eu 3+ Labeled H00025816-A01 antibody (anti-SCC antibody), with Tb 3+ Labeled M86306M antibody (anti-CA125 antibody), with Sm 3+ Labeled MAM02-881 antibody (anti-CEA antibody), with Dy 3+ Labeled 10R-7751 antibody (anti-β-HCG antibody) mixed antibody composed of four monoclonal antibodies; analysis buffer; shared fluorescence enhancer; washing solution; four standard mixtures of SCC, CA125, CEA, and β-HCG Consists of quality controls. The kit also includes negative controls, positive controls, substrates and other related reagents.
[0034] In the kit provided in this example, anti-SCC antibodies H00006317-M01 and H00025816-A01 were purchased from Abn...
Embodiment 2
[0047] The application of the kit provided in Example 1 of the present invention in the comprehensive detection of uterine cancer tumor markers SCC, CA125, CEA, and β-HCG, that is, the kit provided in Example 1 of the present invention is used to comprehensively detect uterine cancer tumor markers at the same time SCC, CA125, CEA, β-HCG time-resolved fluorescent immunoassay method, the reaction principle is as follows figure 1 As shown, the double-antibody sandwich method is used, including the following steps:
[0048] (1) Dilute the four protein standard mixtures of SCC, CA125, CEA, and β-HCG with the analysis buffer, that is, the quality control product, and make a series of concentration gradients containing the four protein standards of SCC, CA125, CEA, and β-HCG The mixed solution of the product;
[0049] (2) Take 100 μl human serum of the sample to be tested and the mixed solution of four protein standards prepared in step (1), and add the mixed antibody package compos...
Embodiment 3
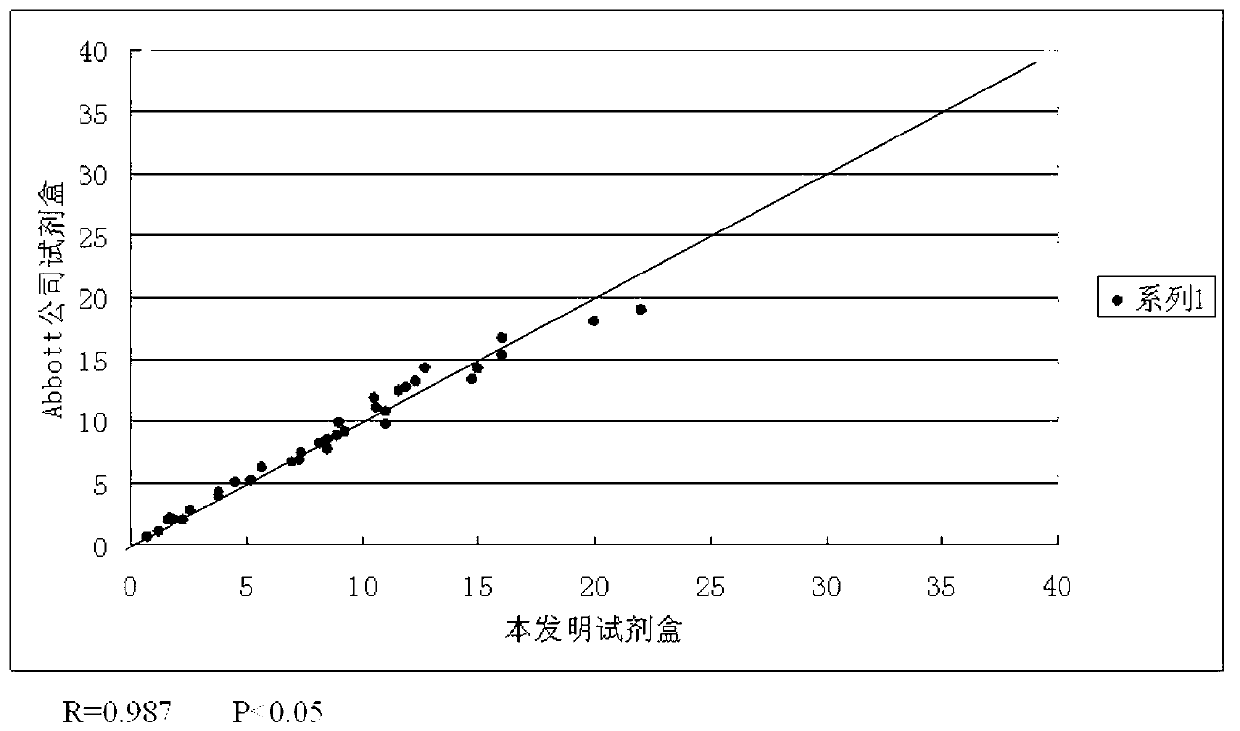
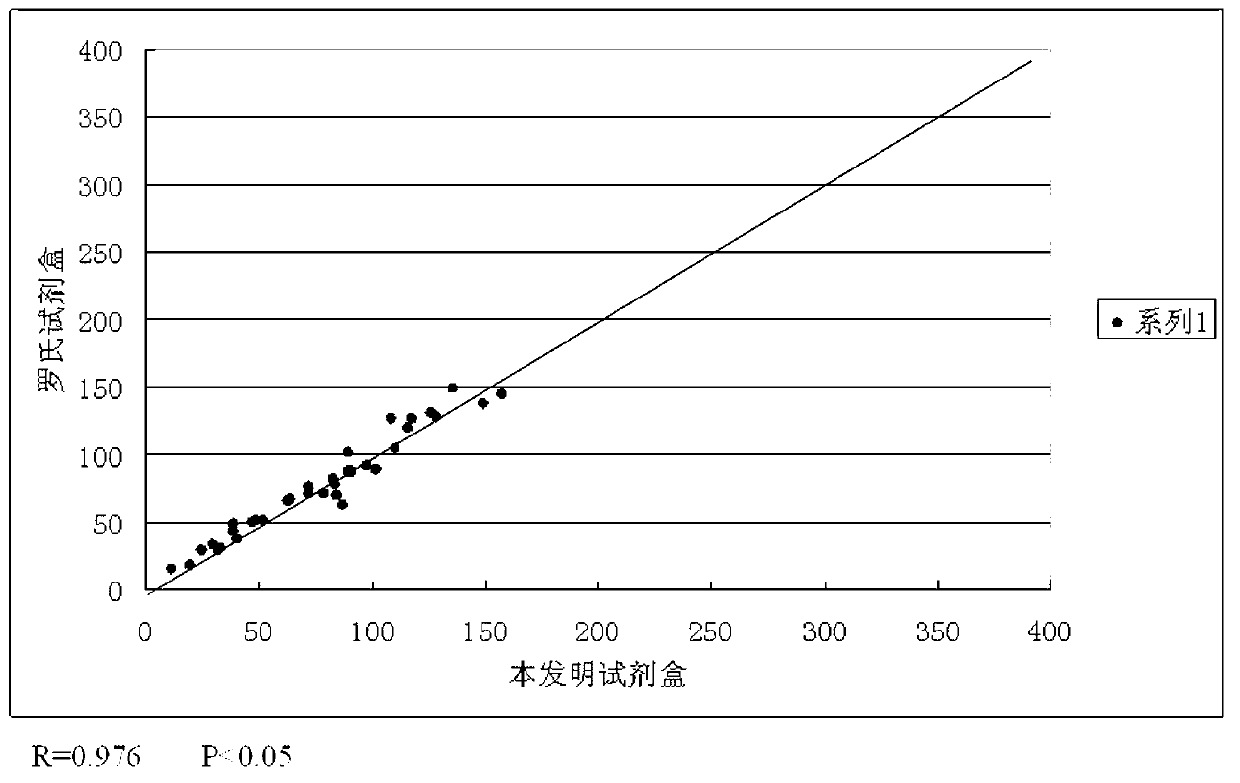
[0051] The numerical values of SCC, CA125, CEA, β-HCG four indexes that test kit of the present invention obtains with 35 serum samples and test SCC with the single indicator kit of Abbott Company respectively with the same 35 serum samples, Roche Company's The data obtained by testing CA125 with a single-index kit, testing CEA with a single-index kit from Abbott, and testing β-HCG with a single-index kit from Siemens were respectively subjected to regression correlation analysis. figure 2 , 3 , 4, 5, as can be seen from the accompanying drawings, each index data obtained by the test kit of the present invention has a good correlation with the data obtained by the test of the single index kit respectively.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap