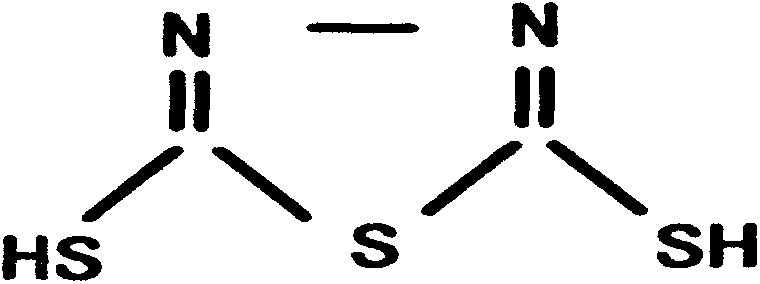
The preparation technology of methylmercaptothiadiazole
A technology of methylmercaptothiadiazole and preparation process, which is applied in the field of medicine and chemical industry, can solve the problems of high production cost, time-consuming, reduced reaction rate, etc., and achieves reduction of addition reaction yield, prevention of excessive temperature rise, and improvement of hydrazine. The effect of chemical reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] (1) Hydrazination reaction: pump 184kg of ethyl acetate and 127.5kg of 80% hydrazine hydrate into a 500L reaction kettle, seal the reaction kettle, start stirring, and slowly heat up to 107°C within one hour after the temperature is constant , keep the temperature for 2.1 hours, transfer the viscous acetylhydrazide solution obtained after cooling to a 1000L reactor;
[0021] (2) Addition reaction: start stirring, and when the temperature of the acetylhydrazide solution is lowered to 10°C, quickly drop 200kg of carbon disulfide into the acetylhydrazide solution, and when the reaction temperature rises to 25°C, start to drop the methanol solution of liquid ammonia. When there is reflux in the device, control the dropping speed, keep the reaction temperature at about 26-32°C, slow down the dropping speed after the light yellow ammonium salt crystallizes out, and keep the reaction material yellow. Check the pH value of the reaction liquid at any time. After the pH=7.5 becom...
example 2
[0026] Except that the molar mass ratio of ethyl acetate to hydrazine hydrate in the hydrazination reaction is 1.3:1, the hydrazination reaction temperature is 104°C, the addition reaction temperature is 24.5°C, and the molar mass ratio of carbon disulfide to acetylhydrazide in the addition reaction is 1.2: Except 1, all the other steps are with example 1, finally obtain the finished product 269.3kg of methylmercaptothiadiazole.
example 3
[0028] Except that the molar mass ratio of ethyl acetate to hydrazine hydrate in the hydrazination reaction is 1.2:1, the hydrazination reaction temperature is 110°C, the addition reaction temperature is 35.5°C, and the molar mass ratio of carbon disulfide to acetylhydrazine in the addition reaction is 1.4: Except 1, all the other steps are with example 1, finally obtain the finished product 270.4kg of methylmercaptothiadiazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com