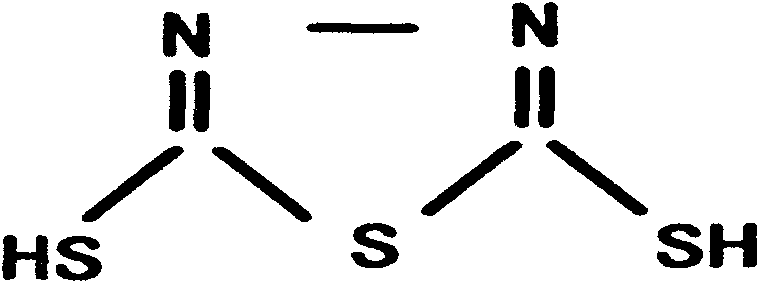
Preparation technology of mercapto-5-methyl-1,3,4-thiadiazole
A technology of methylmercaptothiadiazole and preparation process, which is applied in the field of medicine and chemical industry, can solve the problems of reducing reaction rate, high production cost, time-consuming and the like, and achieves improvement of hydrazation reaction rate, reduction of addition reaction yield, and prevention of temperature The effect of rising too fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] (1) Hydrazine reaction: 184kg of ethyl acetate and 127.5kg of 80% hydrazine hydrate are pumped into a 500L reactor, the reactor is sealed, and the stirring is started. After the temperature is constant, the temperature is slowly raised to 107℃ within one hour , Constant temperature for 2.1 hours, after cooling, transfer the obtained viscous acethydrazine solution to a 1000L reactor;
[0021] (2) Addition reaction: start stirring, and when the temperature of the acetylhydrazine solution is lowered to 10°C, 200kg of carbon disulfide is quickly added dropwise to the acetylhydrazine solution. When the reaction temperature rises to 25°C, the methanol solution of liquid ammonia is added dropwise. When the device is refluxed, the dropping rate is controlled to keep the reaction temperature at about 26-32°C. After the light yellow ammonia salt crystallizes, the dropping rate is slowed down and the reaction material is yellow. If the block is dropped, the reaction liquid will be red...
example 2
[0026] Except that the molar mass ratio of ethyl acetate to hydrazine hydrate in the hydrazation reaction is 1.3:1, the hydrazation reaction temperature is 104°C, the addition reaction temperature is 24.5°C, and the molar mass ratio of carbon disulfide to acetylhydrazine in the addition reaction is 1.2: Except for 1, the remaining steps are the same as in Example 1, and finally 269.3 kg of methyl mercaptothiadiazole is obtained.
example 3
[0028] Except that the molar mass ratio of ethyl acetate to hydrazine hydrate in the hydrazation reaction is 1.2:1, the hydrazation reaction temperature is 110°C, the addition reaction temperature is 35.5°C, and the molar mass ratio of carbon disulfide to acetylhydrazine in the addition reaction is 1.4: Except for 1, the remaining steps are the same as in Example 1, and finally 270.4 kg of methyl mercaptothiadiazole is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com