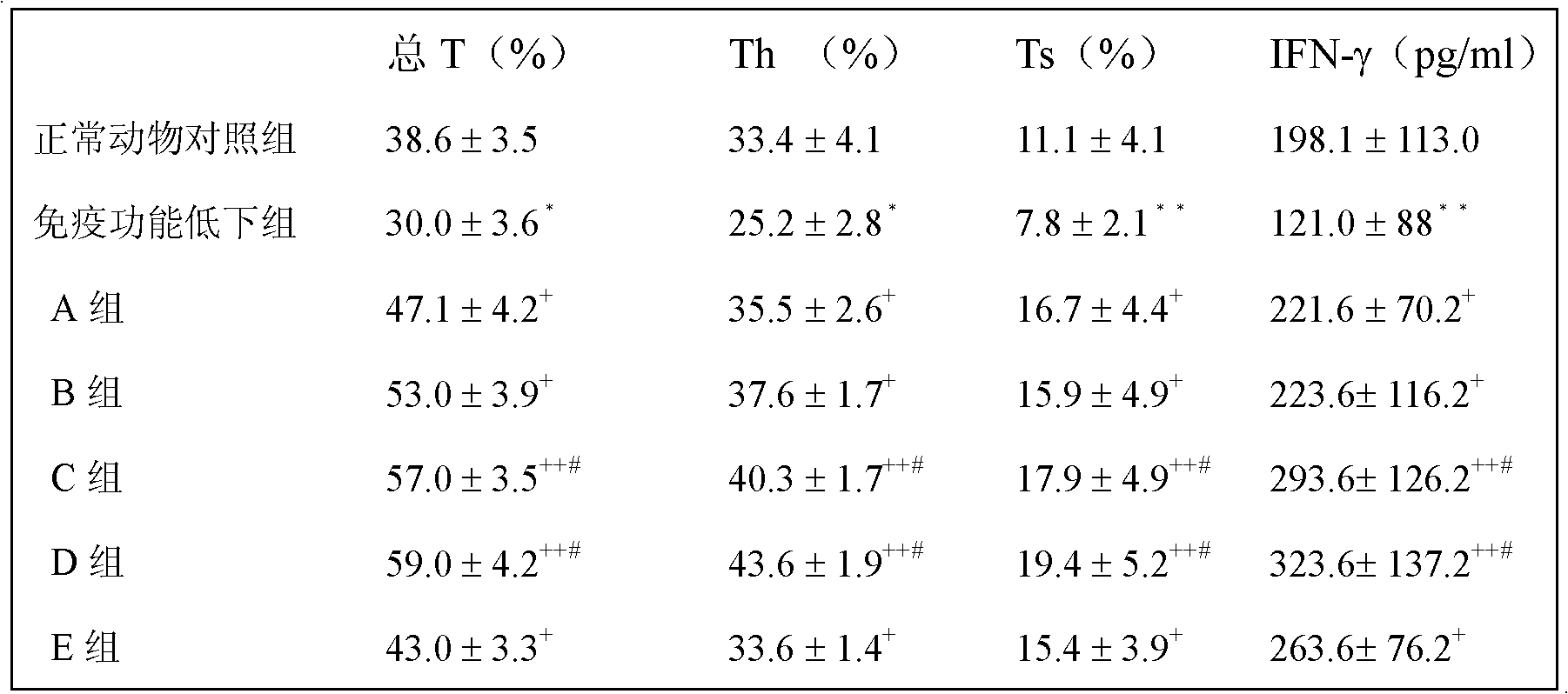An immunomodulator composition, and a pharmaceutical composition and applications thereof
A technology of immunomodulator and composition, applied in the field of immunomodulator composition and its pharmaceutical composition, can solve the problems of insignificant therapeutic effect and long-lasting immunomodulatory effect, and reduce the outbreak and pandemic of infectious diseases Risk, the effect of enhancing the ability of non-specific immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
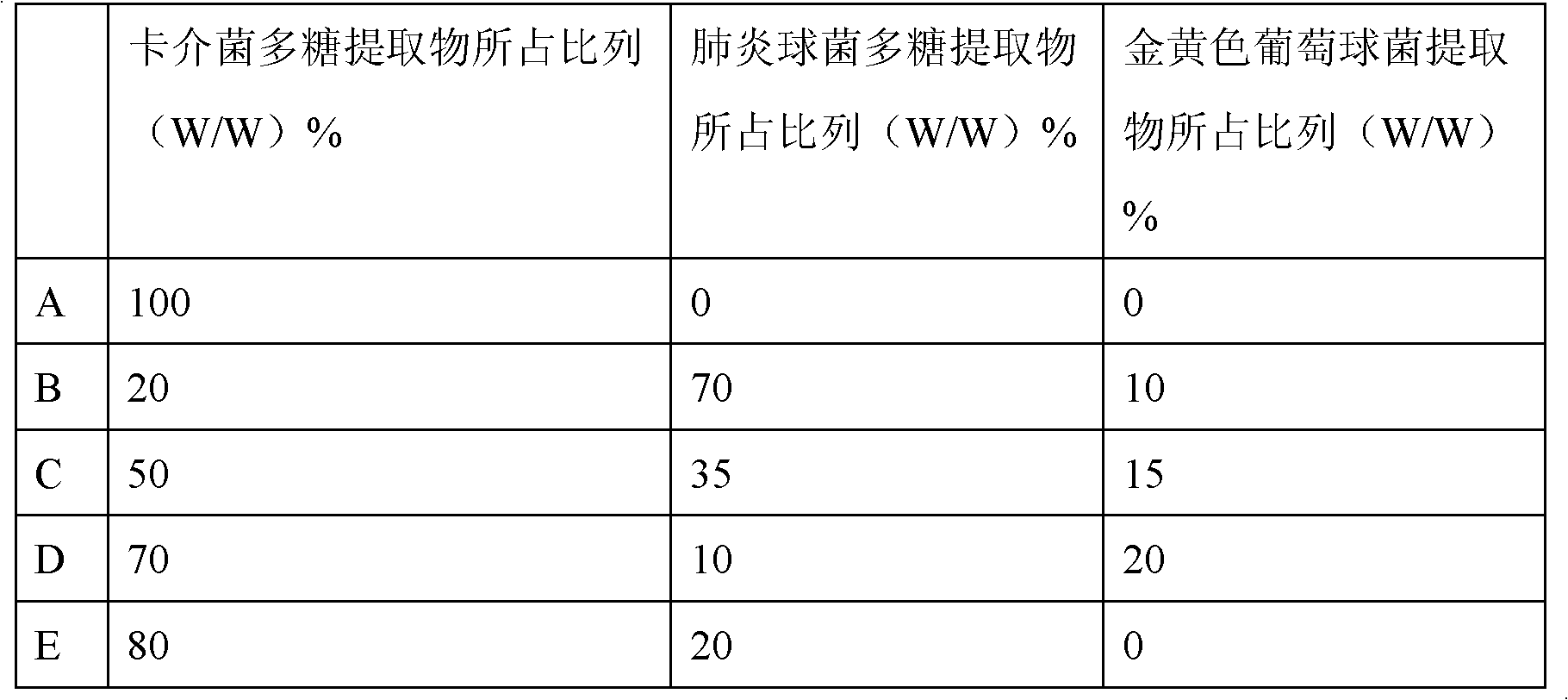
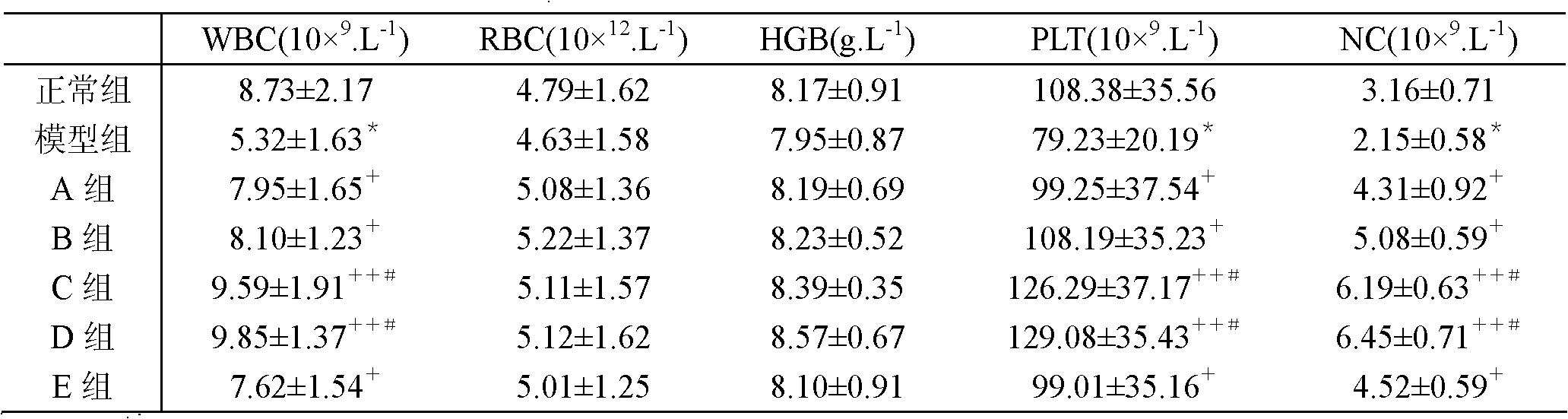
Embodiment 1
[0038] Embodiment 1: the cultivation of BCG and the preparation of polysaccharide extract
[0039] 1. Bacterial culture: Dissolve the strains preserved in liquid cryogenic temperature (BCG strain D2PB302 for the preparation of BCG in China, provided by the Bacteria Room of China Institute for the Control of Pharmaceutical and Biological Products) at room temperature, inoculate them in Potato Sutong medium, and culture continuously at 37°C 14-20 days; or after continuous cultivation at 37°C for 15 days, transfer to improved liquid Sutong medium, and continuous cultivation at 37°C for 14-20 days.
[0040] Wherein, the preparation method of potato Sutong culture medium can be:
[0041] Take washed fresh potatoes (1) and puncture them into cylinders with a piercer, then cut them into 4 cm long slopes with a knife, rinse the potato slopes with running drinking water for 1 hour, and then rinse the potato slopes with purified water; then use Sutong culture medium Rinse the ramp bloc...
Embodiment 2
[0064] Embodiment 2: the cultivation of pneumococcus and the extraction of polysaccharide
[0065] 1. Cultivation of pneumococci: Culture bottles and fermenters can be used for cultivation, and the cultivation conditions are the pneumococcus cultivation conditions known in the art.
[0066] Inoculate 1, 5, 6B, 14, 18C, 19F and 23F serotype freeze-dried strains of pneumococcus on 10% defibrated sheep blood common agar medium, at 36°C, 5% CO 2 In the environment, the 2nd generation liquid medium is propagated after static culture, and the inoculum size is about 10 8 CFU / mL was inoculated in a fermenter with a certain volume, and cultured with stirring at 36°C. After 2 hours of culture, samples were taken every 1 hour, and the OD value was measured at a wavelength of 600nm. mol / L NaOH was used to adjust the pH to 7.5, and compressed air was fed intermittently, and the culture was terminated when the OD value reached 1.5-1.7 (about 7-9 hours). Bacteria were inactivated with (V / V...
Embodiment 3
[0071] Embodiment 3: the cultivation of staphylococcus aureus and the extraction of polysaccharide
[0072] 1. Cultivation of Staphylococcus aureus: Culture bottles and fermenters can be used for cultivation, and the cultivation conditions are the pneumococcus cultivation conditions known in the art.
[0073] Inoculate the freeze-dried strains of Staphylococcus aureus of C5 and C8 serotypes on solid medium for activation, culture at 37°C for 18 hours, and culture the culture on new agar culture at 37°C for 4 hours in order to obtain the exponential growth phase of microorganisms. Then in basic liquid medium (1g / L glucose, 120mg / L Fecl 3 ·6H 2 O, 400mg / LMgcl 2 ·6H 2 O, 10mg / L CaCl 2 2H 2 O, 5mg / LZnCl 2 , 58.5g / L NaCl, 6g NH 4 Cl, 6.8g NH 4 Cl, pH6.2~7.5, optimally 6.5) for cultivation. After culturing for 2 hours, samples were taken every 1 hour, and the OD value was measured at a wavelength of 600nm. 600 When ≥1.5, terminate the culture.
[0074] The culture was in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com