Butylphthalide sublingual tablet and preparation method thereof
A kind of technology of butylphthalide and composition, applied in the field of sublingual tablet of butylphthalide and preparation thereof, can solve the problem that the preparation of sublingual oral preparations is not retrieved and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
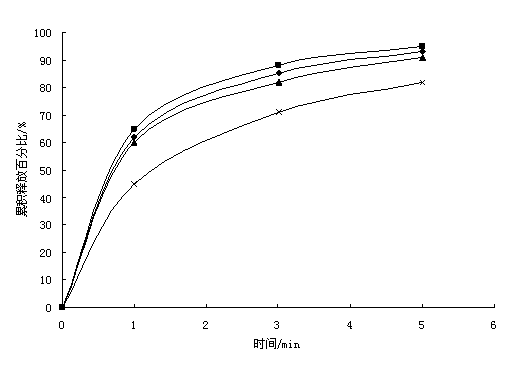
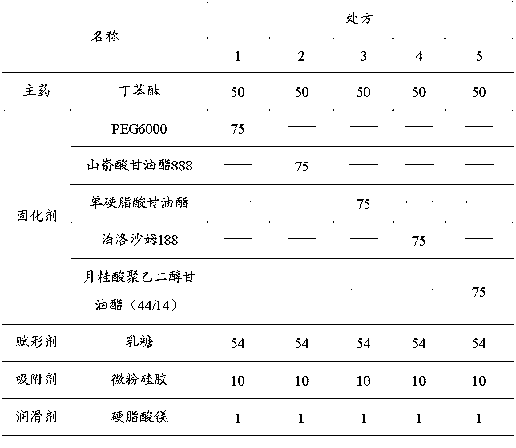
Image
Examples
Embodiment 1
[0043]
[0044] Preparation method: add butylphthalide, macrogol glyceride laurate and lactose into a twin-screw extruder to extrude. The temperature of the extruder section is set at 30°C-60°C-60°C-60°C-45°C, and the screw speed of the host machine and the speed of the feeder are both set at 25rpm. The extrudate is cooled and cut into pieces.
Embodiment 2
[0046]
[0047] Preparation method: add butylphthalide, macrogol glyceride laurate and lactose into a twin-screw extruder to extrude. The temperature of the extruder section is set at 30°C-60°C-60°C-60°C-45°C, and the screw speed of the host machine and the speed of the feeder are both set at 25rpm. The extruded product is cooled and pulverized into granules, added with micropowdered silica gel and magnesium stearate, and pressed into tablets with a hardness of 4 to 5 kg.
Embodiment 3
[0049]
[0050] The preparation method is the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com