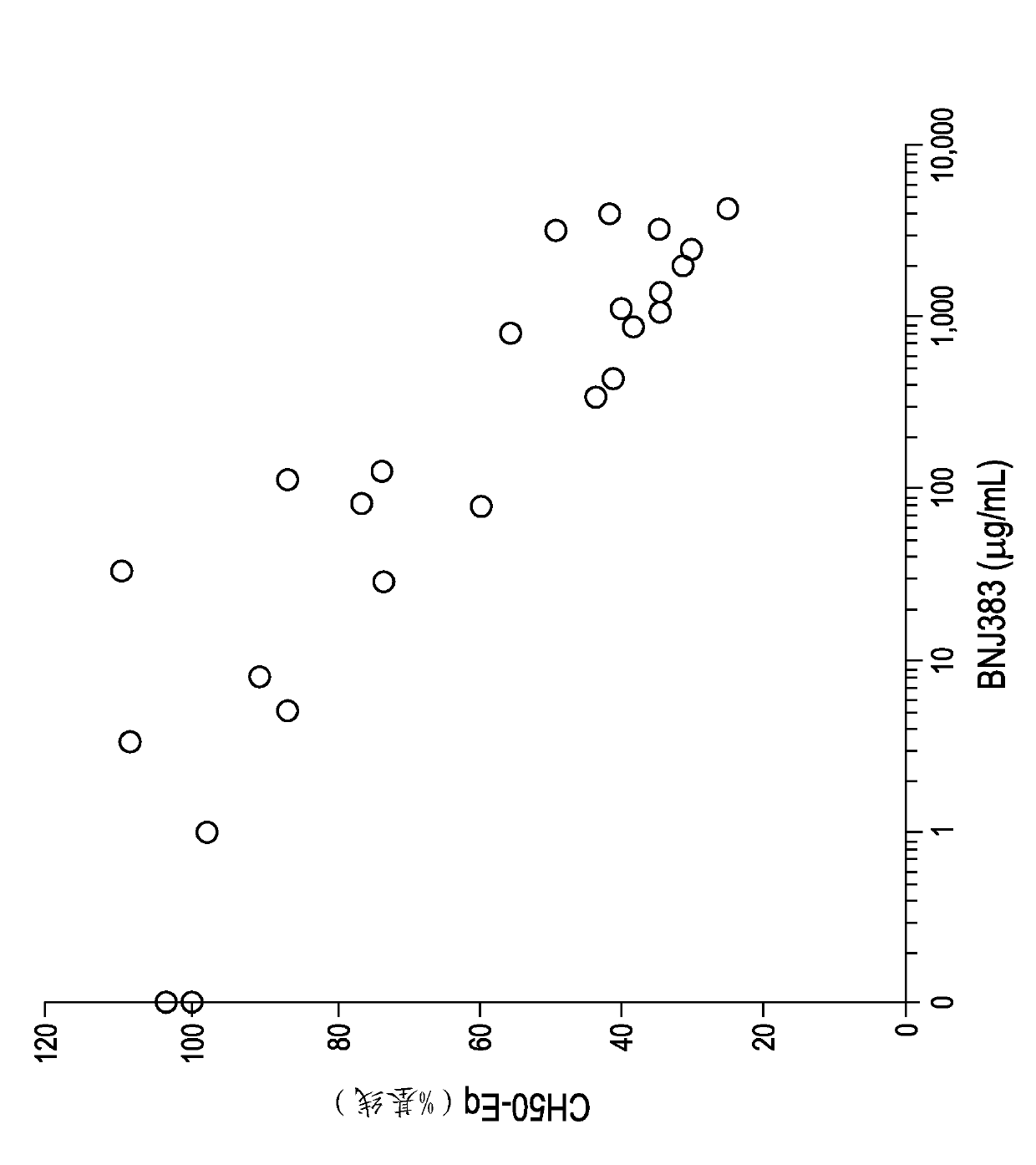
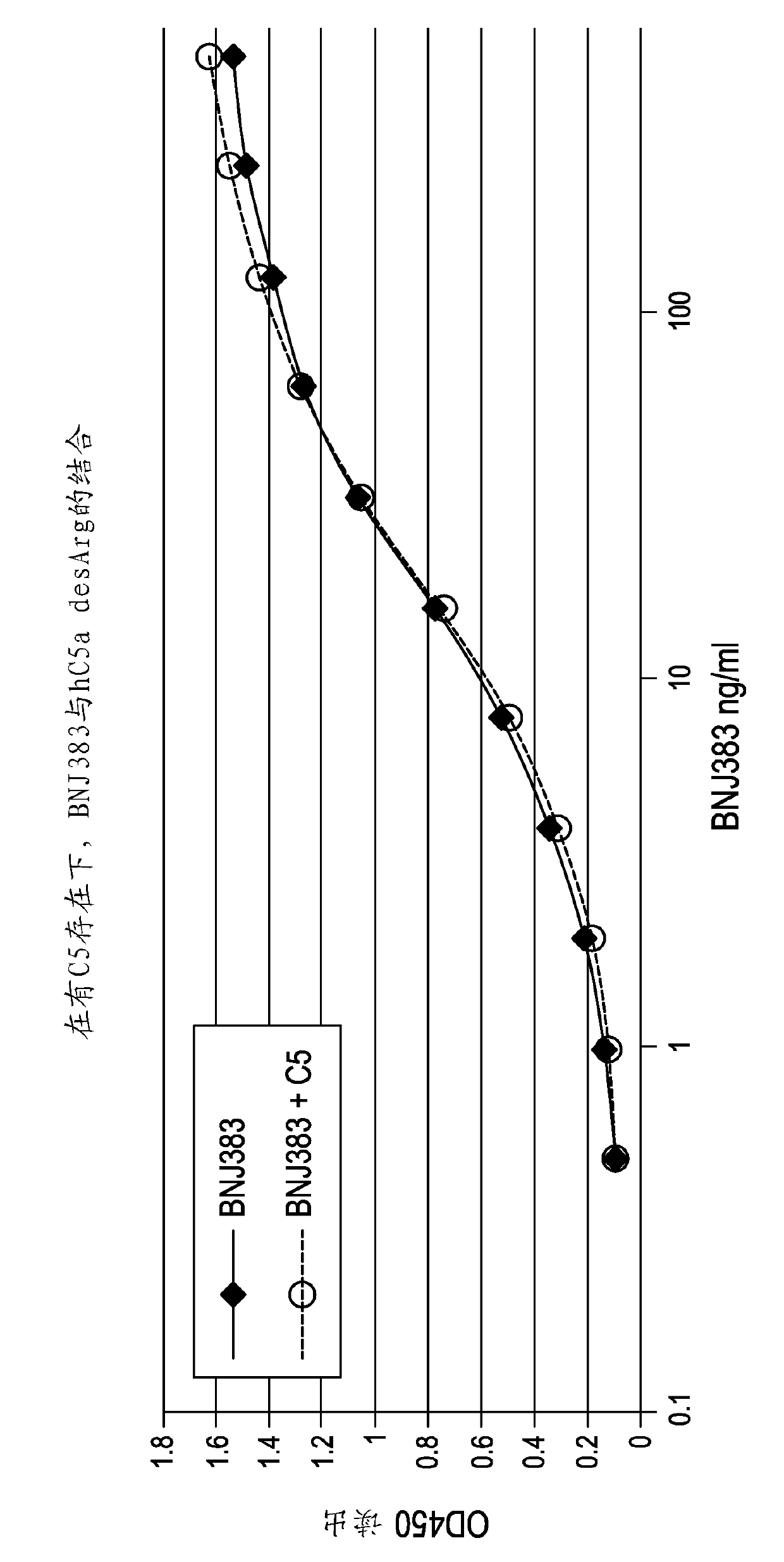
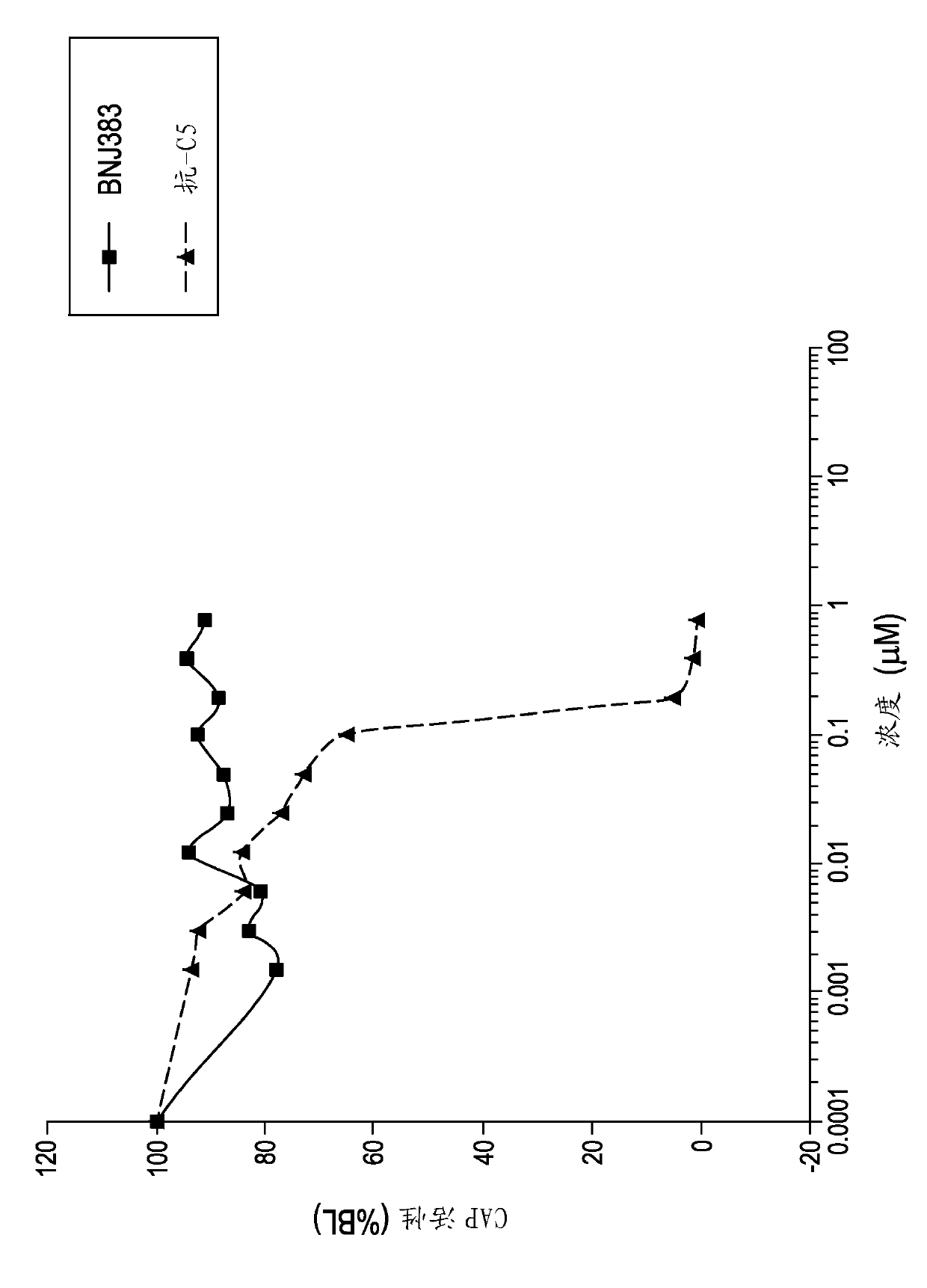
Anti-c5a antibodies and methods for using the antibodies
A technology of antibodies and antigens, applied in chemical instruments and methods, antibodies, antibody medical components, etc., can solve problems such as inability to form C5 converting enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0400] Example 1. Immunization method
[0401] The anti-C5a antibody of the present invention is a humanized form of murine antibody prepared under the following immunization protocol. Immunizations producing antibodies against human desarginated C5a were performed on 4 mice, including 2 mice of the DBA / 2J strain and 2 mice of the A / J strain. These lines were chosen because they carry alleles Hc 0 , which would render them deficient in endogenous C5. All immunizations were repeated at 14 day intervals for a total of 3 immunizations. Approximately 14 days after the last immunization and 5–7 days before harvest, all animals received a subcutaneous booster immunization of approximately 50 μg of purified C5a (in 200 μL of adjuvant emulsion). Using an ELISA assay, titration of sera from immunized mice confirmed that the mice exhibited a strong antibody response against the human desarginated C5a immunogen.
Embodiment 2
[0402] Example 2. Determination of the specificity of mouse antibodies to human C5a
[0403]A panel of 5 mouse anti-human C5a Fabs, representative of neoepitope-selective Fabs, were converted into full-length mouse IgG2a antibodies, named: 5an048ME, 5an101ME, 5an178ME, 5an179ME and 5an180ME. The specificity of these antibodies was evaluated using the Biolayer Interferometry method on Octet (ForteBio Inc.) (the amino acid sequences of the light and heavy chain CDR sets of each antibody are described in Table 3, as defined by Kabat). Briefly, human C5a, human C5a des Arg, human full-length C5, or the C5a paralog human C3a and human C4a were passed through the amine group with a stoichiometry of < 1 (biotin):1 (antibody) The clusters are conjugated with biotin and immobilized on streptavidin tips. The loaded tips were then exposed to a solution containing 20 nM anti-C5a IgG antibody. Each antibody binds C5a and desarginated C5a. Anti-C5a IgG antibodies bind neither C3a nor C...
Embodiment 3
[0411] Example 3. Humanization of selected mouse anti-human C5a antibodies
[0412] The variable regions of 2 related mouse anti-human C5a antibodies (5an101ME and 5an185ME) were selected for humanization into full-length IgG antibodies. Humanization of the light and heavy chain variable regions is based on the identification of a single framework region from a human antibody (preferably a germline v-gene) with a high degree of sequence identity to the original murine parent antibody. Methods for identifying suitable candidate framework regions are described in US Patent No. 7,393,648 to Rother and Wu. Frameworks (FW) and complementarity determining regions (CDRs) were defined according to the method described by Kabat, Chothia and IMGT® (International ImmunoGenetics Information System; France). Briefly, database queries were performed independently for light and heavy chain variable regions using various antibody fragments including: complete murine variable regions from F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com