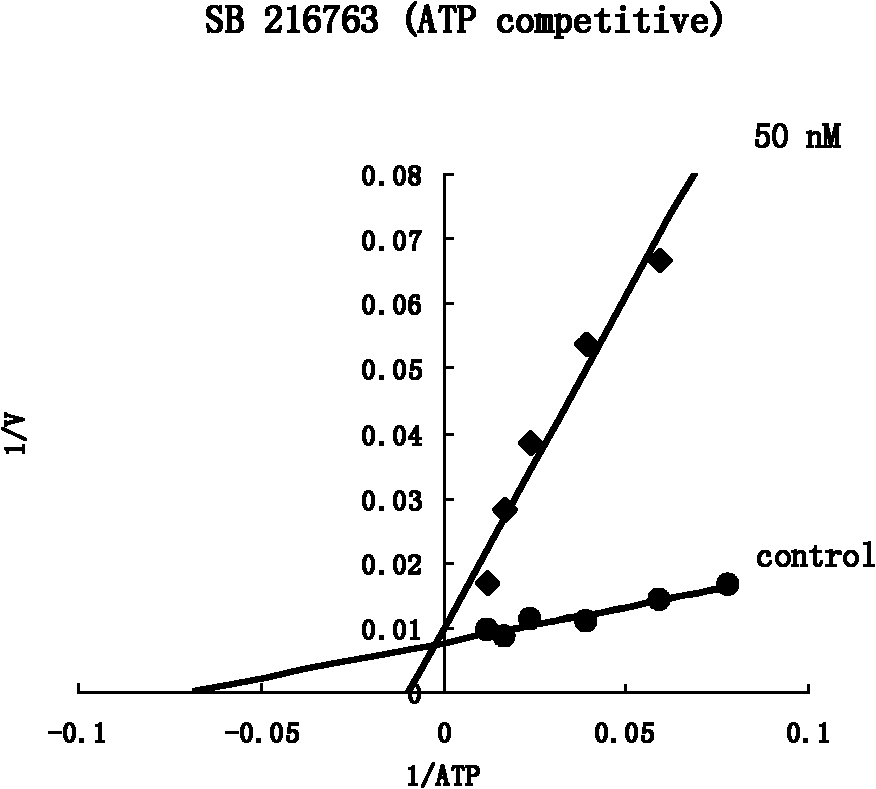
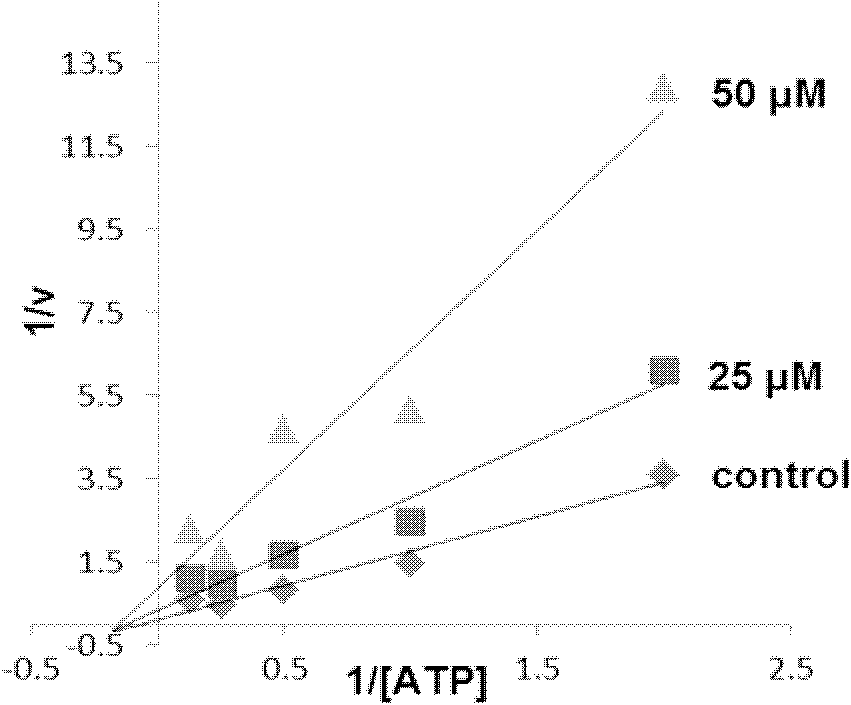
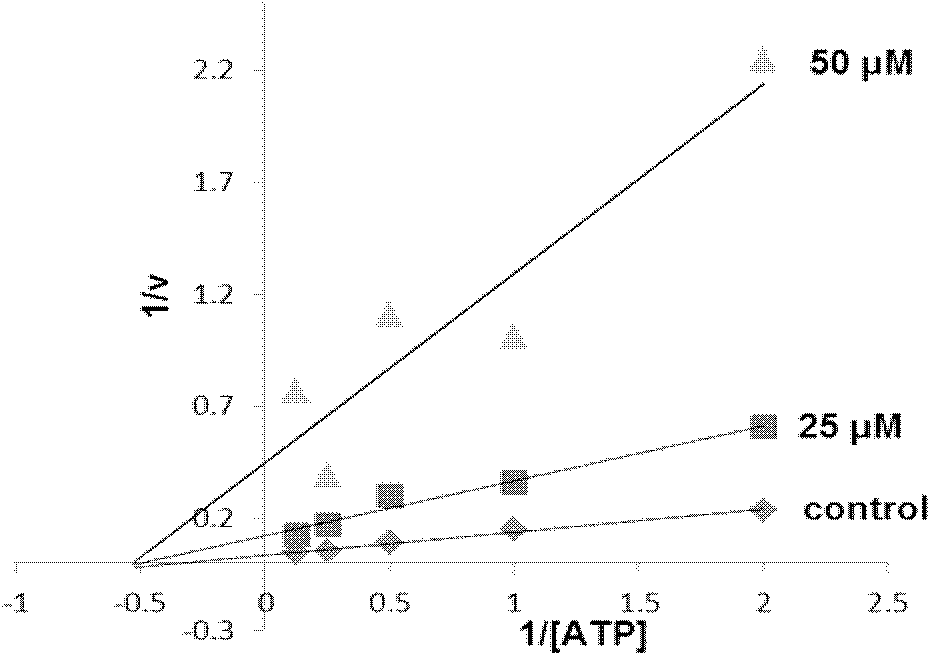
2,3-dihydrobenzazapine*compound or its salt and its medical application
A technology of heterocompounds and dihydrobenzene, which is applied in drug combination, organic chemistry, antineoplastic drugs, etc., and can solve problems such as multiple side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 5-(2-nitrobenzyl)-2,3-dihydro-2-phenyl[1,5]benzothiazepine -4-keto (LQaa)
[0038] (a). 2,3-dihydro-2-(2-phenyl)[1,5]benzothiazepine Preparation of -4(5H)-one (ZPa01)
[0039]
[0040] Cinnamic acid (28.4 g, 0.106 mol), o-aminothiophenol (13.3 g, 0.106 mol), and an appropriate amount of molecular sieves were reacted at 190° C. for 6.5 hours. Molecular sieves were removed by filtration, and the filtrate was cooled to precipitate a white solid, which was recrystallized from acetonitrile to obtain a white crystal with a yield of 25%. m.p.: 177.6-179.9°C. 1 H NMR (400MHz, CDCl 3 )δ: 8.30 (bd, 1H, N H ), 7.42-7.17 (m, 9H, PhH), 4.91-4.87 (m, 1H, C H -CH 2 ); 2.94-2.79 (m, 2H, CH-C H 2 ).MS (+ESI) m / z: 256.1 (M+H) + .
[0041] (b) Preparation of 5-(2-nitrobenzyl)-2,3-dihydro-2-phenyl[1,5]benzothiazepine -4(5H)-Kone (LQaa)
[0042]
[0043] Add compound ZPa01 0.255g (1mmol) and 60% NaH 0.0900g (3mmol) to 4ml reevaporated DMF, after stirring...
Embodiment 2
[0045] Preparation of 5-(3-nitrobenzyl)-2,3-dihydro-2-phenyl[1,5]benzothiazepine -4(5H)-Kone (LQac)
[0046]
[0047] The preparation method is the same as above LQaa, wherein 3-nitrobenzyl bromide is used instead of 2-nitrobenzyl bromide, 0.359 g of white solid, yield 92%. m.p.196.5-198.0. 1 H NMR (400MHz, CDCl 3 )δ: 8.17-8.07 (d, 2H, PhH), 7.74-7.13 (m, 11H, PhH), 5.58-5.54 (d, 1H, Ph-C H 2 ), 4.90-4.88 (m, 1H, Ph-C H 2 ), 4.87-4.84 (m, 1H, Ph-C H ), 2.96-2.83 (m, 2H, CH-C H 2 ).MS(+ESI)m / z: 390.9(M+H)+.
Embodiment 3
[0048] Example 3: Preparation of 5-(2-bromobenzyl)-2,3-dihydro-2-phenyl[1,5]benzothiazepine -4(5H)-Kone (LQad)
[0049]
[0050] The preparation method is the same as above LQaa, wherein 2-bromobenzyl bromide is used instead of 2-nitrobromobenzyl, 0.387 g of white solid, yield 91%. m.p.167.6-170.4. 1 H NMR (400MHz, CDCl 3 )δ: 7.62-7.07 (m, 13H, PhH), 5.25-5.23 (m, 2H, Ph-C H 2 ), 4.92-4.87 (m, 1H, Ph-C H ), 2.98-2.87 (m, 2H, CH-C H 2 ).MS(+ESI)m / z: 424.0(M+H)+, 426.4(M+H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com