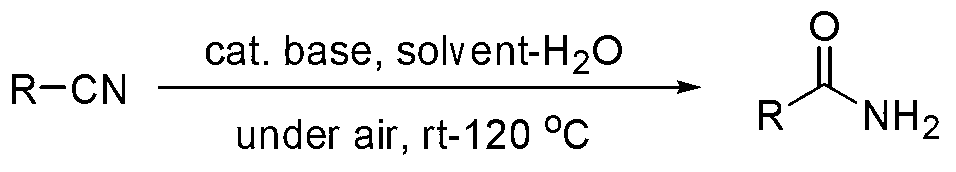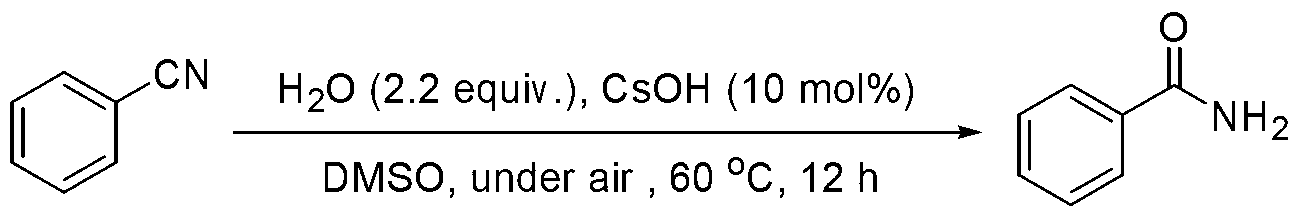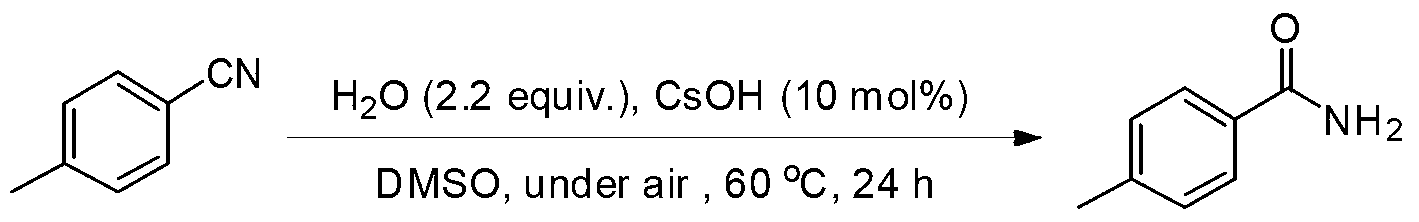Environmental-friendly nitrile hydrolysis method
A green, alkaline catalyst technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of large amount of catalyst, poor catalyst effect, high toxicity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of benzamide from benzonitrile
[0020]
[0021] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), PhCN (1.0mmol) and H 2 O (0.04mL, 2.2equiv.), then add DMSO (0.5mL) as solvent, seal the reaction tube and heat to 60oC for 12h. The reaction conversion rate was over 99% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 94%. 1 HNMR (500MHz, d 6 -DMSO): δ8.01(b,1H),7.88-7.86(m,2H),7.54-7.51(m,1H),7.47-7.44(m,2H),7.39(b,1H). 13 CNMR (125.4MHz, d 6 -DMSO): δ168.0, 134.1, 131.2, 128.2, 127.4. MS (EI): m / z (%) 224 (6), 223 (32), 222 (14), 132 (2), 131 (8), 130(4),118(2),106(23),105(100),104(5),103(9),91(5),79(9),78(4),77(11), 65(3).
Embodiment 2
[0023] Preparation of p-toluamide from p-methylbenzonitrile
[0024]
[0025] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), p-tolunitrile (1.0mmol) and H 2 O (0.04mL, 2.2equiv.), then add DMSO (0.5mL) as solvent, seal the reaction tube and heat to 60oC for 24h. The reaction conversion rate was over 91% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 85%. 1 HNMR (500MHz, d 6 -DMSO):δ7.71(d,J=8.0Hz,2H),7.25(m,3H),6.08(b,1H),5.93(b,1H),2.40(s,3H). 13 CNMR (125.4MHz, d 6 -DMSO): δ169.6, 142.6, 130.6, 129.4, 127.5, 21.6. MS (EI): m / z (%) 136 (5), 135 (60), 120 (8), 119 (100), 117 (3 ),92(5),91(74),90(8),89(10),65(33),64(4),63(13),51(9),50(6),44(12 ),41(5),40(9),39(20),38(4).
Embodiment 3
[0027] Preparation of m-methylbenzamide from m-methylbenzonitrile
[0028]
[0029] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), m-tolunitrile (1.0mmol) and H 2 O (0.04mL, 2.2equiv.), then add DMSO (0.5mL) as solvent, seal the reaction tube and heat to 60oC for 24h. The reaction conversion rate was over 99% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 85%. 1 HNMR (500MHz, d 6 -DMSO): δ7.65(s,1H),7.59(s,1H),7.34(s,1H),7.27(s,1H),6.15(b,2H),2.40(s,3H). 13 CNMR (125.4MHz, d 6 -DMSO): δ169.8, 138.5, 133.4, 132.7, 128.5, 128.1, 124.3, 21.3. MS (EI): m / z (%) 136 (6), 135 (63), 120 (9), 119 (100) ,117(4),116(2),92(6),91(77),90(5),89(7),65(18),63(6),62(2),51(4) ,44(3),39(6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com