Method and equipment for preparing methyl carbamate
A technology of methyl carbamate and ammonia, which is applied in the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as equipment corrosion, reduce carbon emissions, be beneficial to environmental protection, and improve The effect of one-way conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
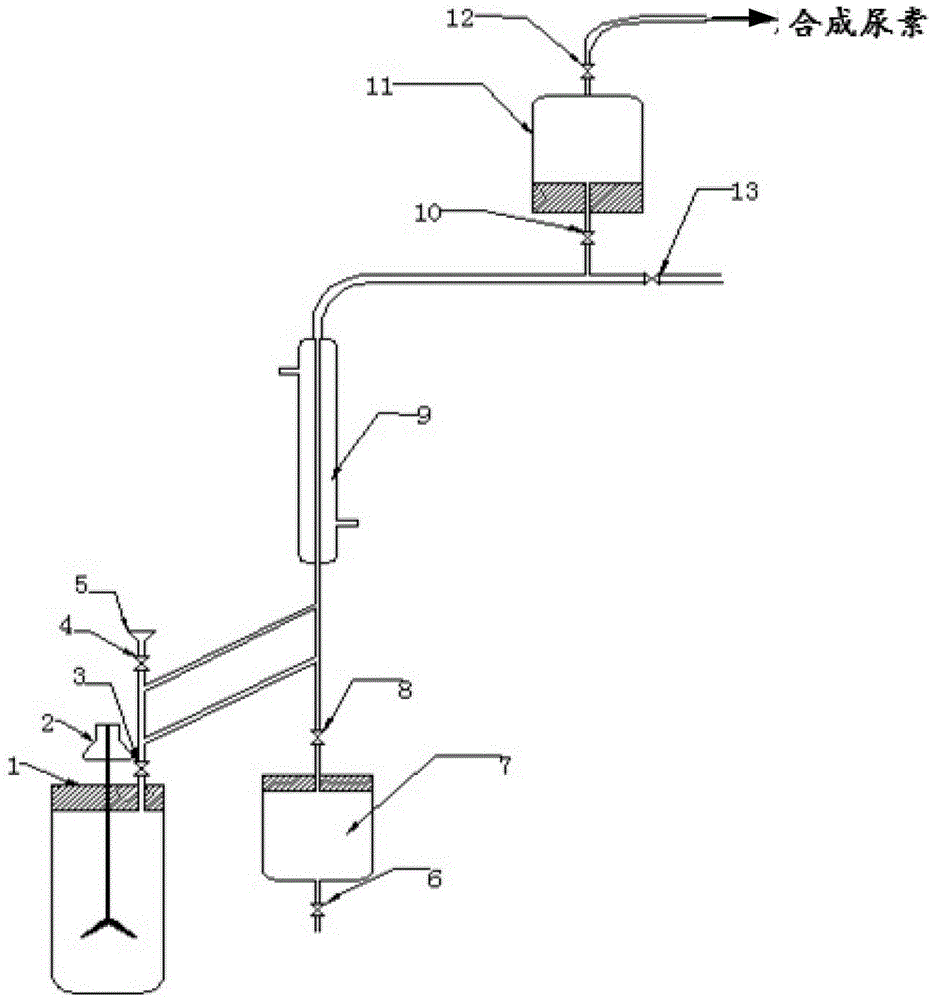
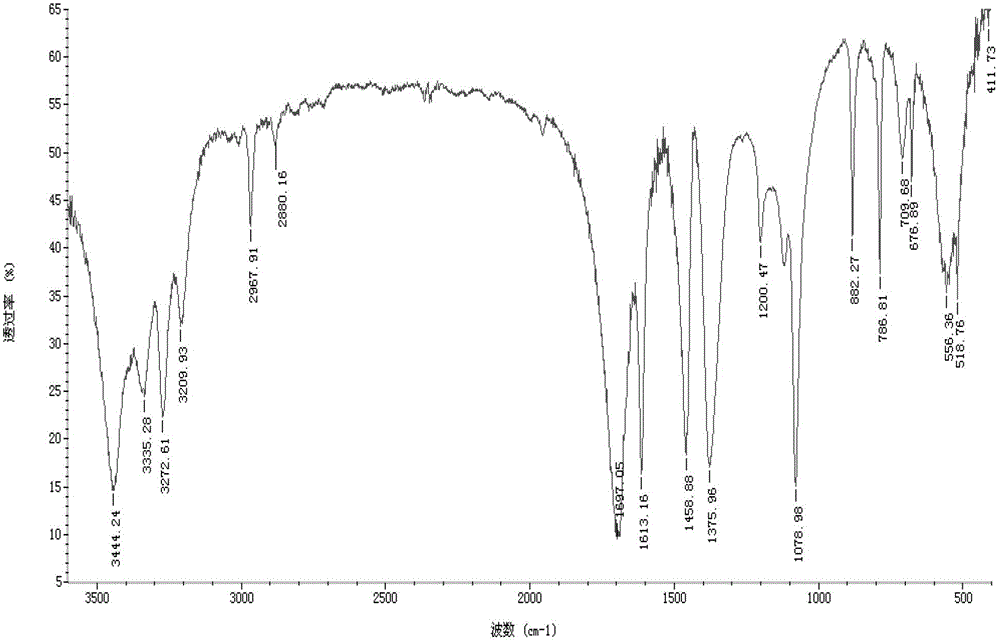
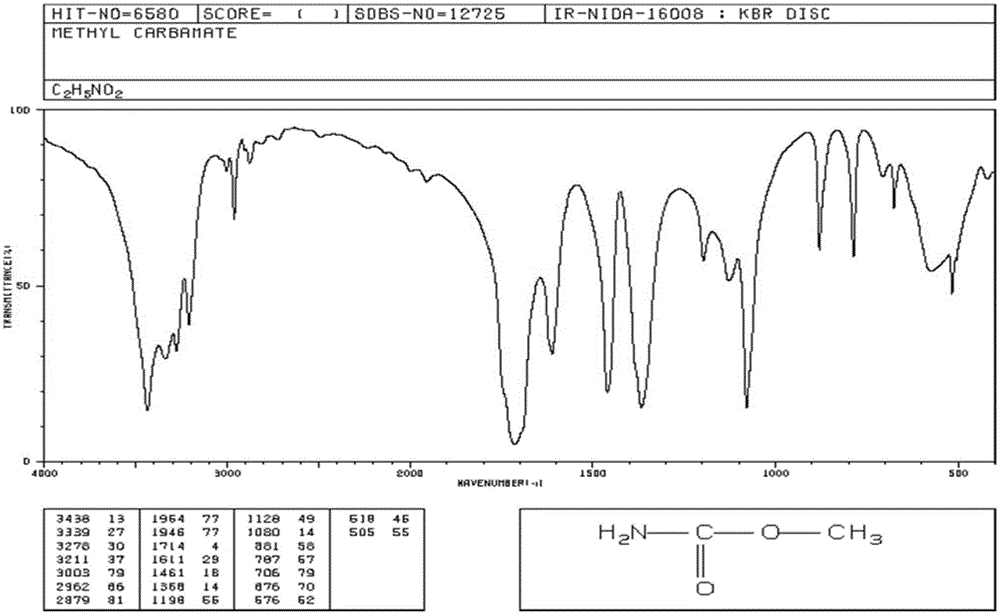
[0043] exist figure 1 In the equipment shown, add 120g of urea and 560ml of methanol into the 1L reactor 1 (the molar ratio of feeding is about 1:7), close all the valves, and stir to dissolve. Heat urea and methanol to 160°C, correspondingly, the pressure inside the reactor 1 will gradually rise to about 1.7MPa, and react under this condition for 3 hours. Then open the valves 10, 12, and then open the valve 3. At this time, the ammonia gas is exported to the ammonia recovery device 11 through the condensing device 9, and the pressure and temperature in the reactor 1 tend to decline. After exporting the ammonia gas, close the valves 10 and 12, open the valve 8, gradually raise the temperature in the reactor 1 to about 100°C, and recover the unreacted methanol to the methanol recovery device 7. The reaction product in the reaction kettle 1 was discharged to another vacuum distillation kettle while it was hot, and 88.5 g of methyl carbamate was obtained after vacuum distillatio...
Embodiment 2
[0045] exist figure 1 In the equipment shown, add 120g of urea and 560ml of methanol into the 1L reactor 1 (the molar ratio of feeding is about 1:7), close all the valves, and stir to dissolve. Heat urea and methanol to 160°C, correspondingly, the pressure inside the reactor 1 will gradually rise to about 1.7MPa, and react under this condition for 3 hours. Then open the valves 10, 12, and then open the valve 3. At this time, the ammonia gas is exported to the ammonia recovery device 11 through the condensing device 9, and the pressure and temperature in the reactor 1 tend to decline. When the temperature drops to 60°C, close the valve 3 and continue heating until the temperature and pressure return to the original level, and react for 3 hours. Repeat this operation to export ammonia gas three times (about 1 hour each time). After exporting the ammonia gas for the last time, close the valves 10 and 12, open the valve 8, heat the temperature of the reactor 1 to about 100°C gra...
Embodiment 3
[0047] exist figure 1 In the equipment shown, add 120g of urea and 560ml of methanol into the 1L reactor 1 (the molar ratio of feeding is about 1:7), close all the valves, and stir to dissolve. Heat urea and methanol to 160°C, correspondingly, the pressure inside the reactor 1 will gradually rise to about 1.7MPa, and react under this condition for 3 hours. Then open the valves 10, 12, and then open the valve 3. At this time, the ammonia gas is exported to the ammonia recovery device 11 through the condensing device 9, and the pressure and temperature in the reactor 1 tend to decline. When the temperature drops to 60°C, close the valve 3 and continue heating until the temperature and pressure return to the original level, and react for 3 hours. Repeated operations in this way, ammonia gas is exported six times (each time takes about 1h). After exporting the ammonia gas for the last time, close the valves 10 and 12, open the valve 8, heat the temperature of the reactor 1 to ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com