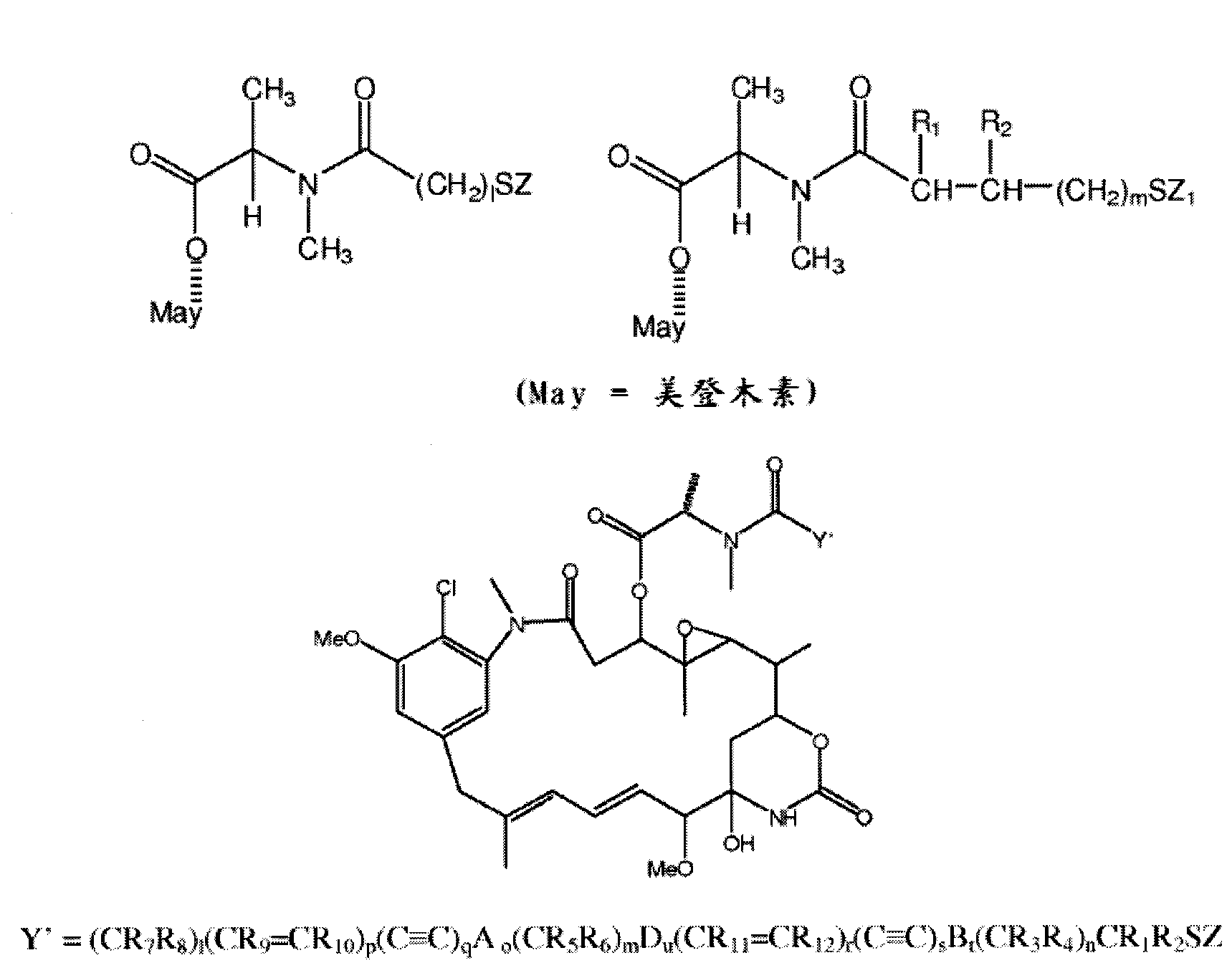
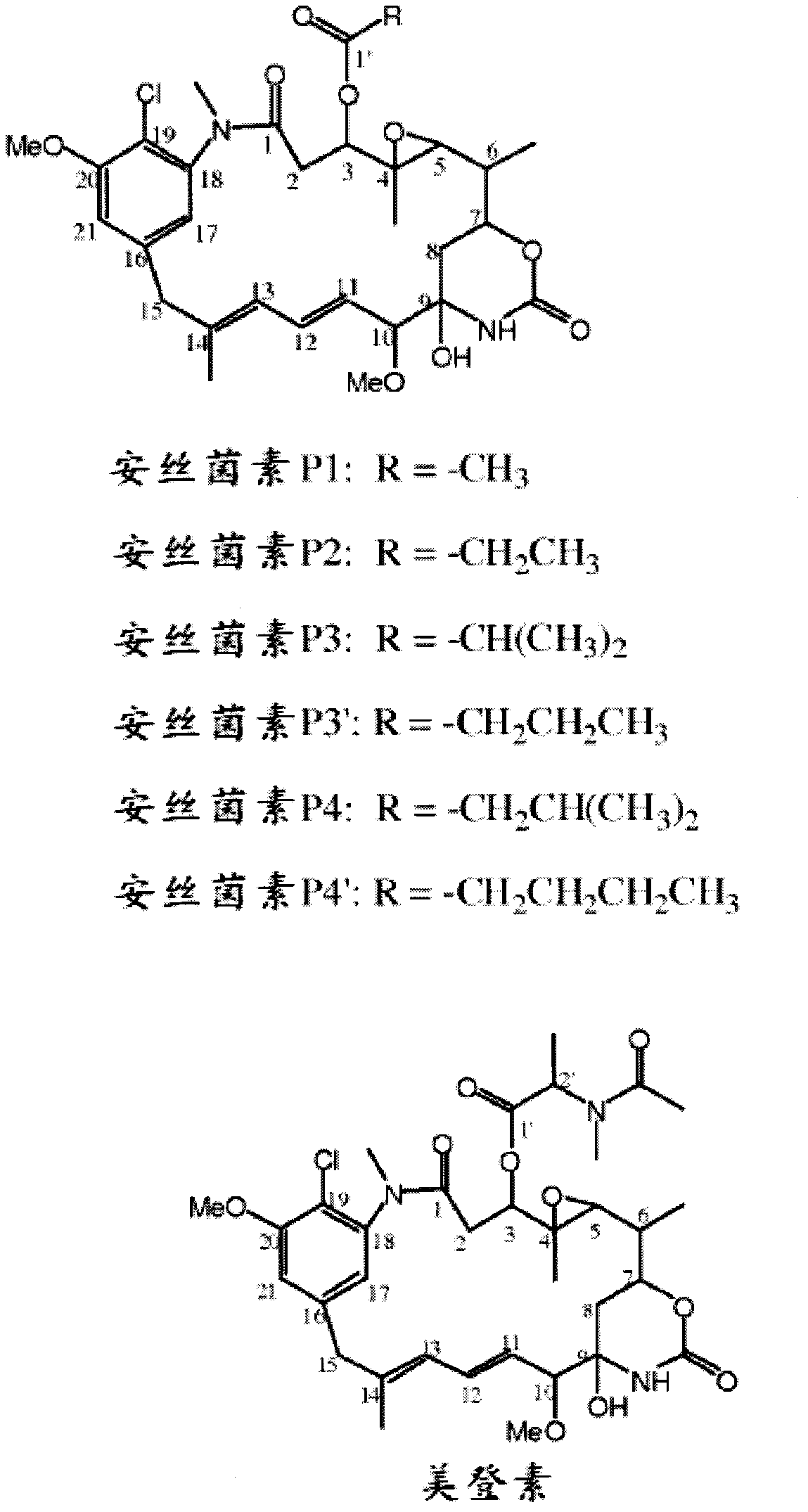
Cytotoxic agents comprising new ansamitocin derivatives
A cell and binding agent technology, applied in the direction of antiviral agent, antifungal agent, non-central analgesic agent, etc., can solve the problem of unreported cancer cell killing activity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
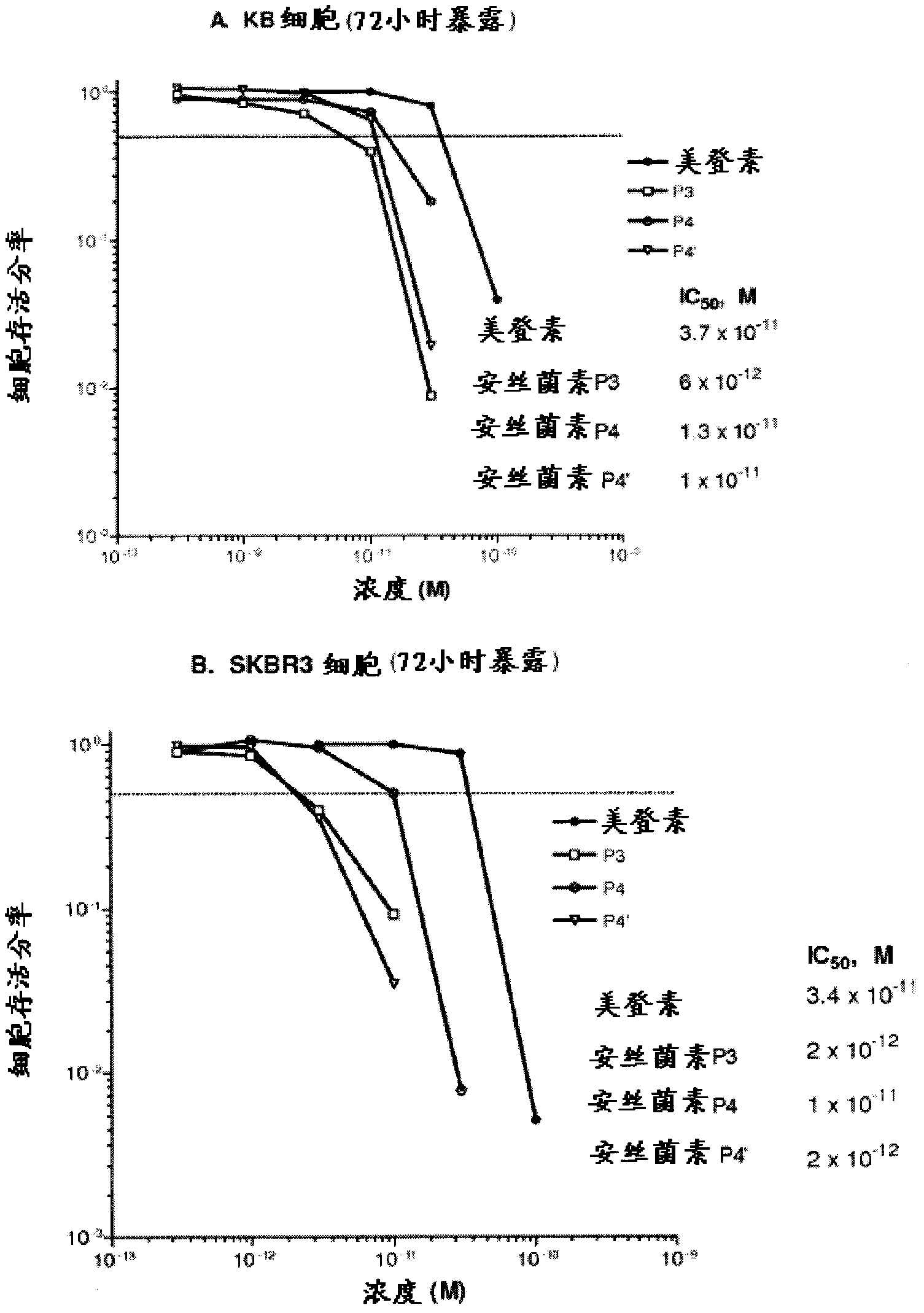
[0348] Example 1. In Vitro Cytotoxicity
[0349]Cytotoxicity studies were performed using clonogen assays. The test cell lines (KB and SK-Br-3) were seeded in 6-well dishes at a constant number of 1000 cells per well. Cells were incubated with various concentrations (0 to 3 nM) of various ansamitocins or maytansines for 72 hours. The medium was then aspirated from the plate and replaced with fresh medium. The medium was allowed to grow, and colonies formed after a total of 7-10 days post inoculation. The medium was then fixed and stained with 0.2% crystal violet in 10% formalin / PBS, and colonies were counted. The seeding efficiency of untreated cells (medium only) was determined by dividing the number of counted colonies by the number of cells seeded. The fraction of drug-exposed cells survived was determined by dividing the number of drug-exposed colonies in wells by the number of colonies in control wells.
[0350] The results of in vitro cytotoxicity measurements of va...
Embodiment 2
[0351] Example 2. Preparation of derivatized carboxylic acids
[0352] Compound 28: 3-Mercaptopropionic acid was reacted with methyl S-methylthiosulfonate in ethanol to afford 28 as described previously. (Widdison, W.C., et al., J Med Chem, 2006.4, 4392-408).
[0353] Compound 30: Compound 30 was prepared by reacting methyl S-methylthiosulfonate with 4-mercapto-1-butanol.
[0354] Compound 32: Compound 32 was prepared by reacting methyl S-methylthiosulfonate with 1-amino-butan-4-thiol (Reineke, T.M. et al. Bioconjugate Chem., 2003 14, 247-254).
[0355] Compound 34: The furan-bearing carboxylic acid 34 was prepared as follows. 5-Hydroxymethyl-2-furoic acid methyl ester (33) (Moore, J.A et al., Journal of Polymer Science, Polymer Chemistry Edition (1984), 22(3) , 863-4) with methanesulfonyl chloride followed by thiourea and hydrolysis to give 33. 33 is then reacted with methylthiosulfonate.
[0356] Compound 37: Compound 37 was prepared by reacting 2,2'-dithiobipyridin...
Embodiment 3
[0359] Embodiment 3. Preparation of 4-nitro-phenol carbonate or 4-nitro-phenol carbamate
[0360] Reaction of 4-nitrophenyl chloroformate with compound 30 affords disulfide-bearing carbonate 43.
[0361] Reaction of 4-nitrophenyl chloroformate with compound 32 affords disulfide-bearing carbamate 44.
[0362] Preparation of Ansamitocin Derivatives
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com