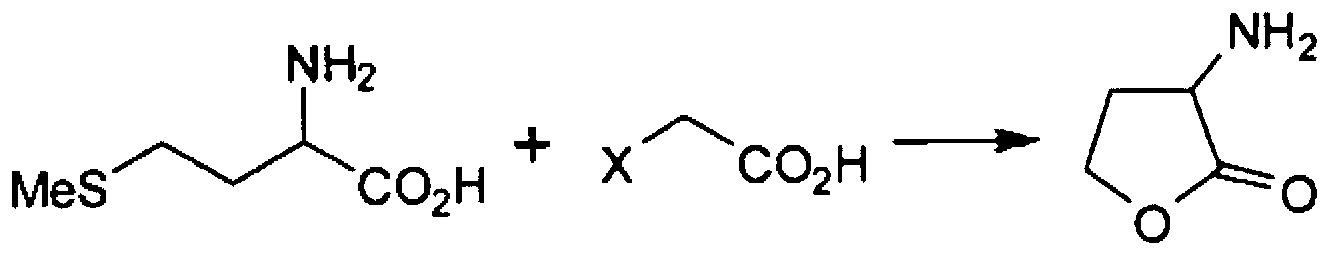Method for producing alpha -mino-gamma-butyrolactone
A manufacturing method, technology of butyrolactone, applied in organic chemistry methods, chemical instruments and methods, asymmetric synthesis, etc., can solve unsatisfactory industrial production methods and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
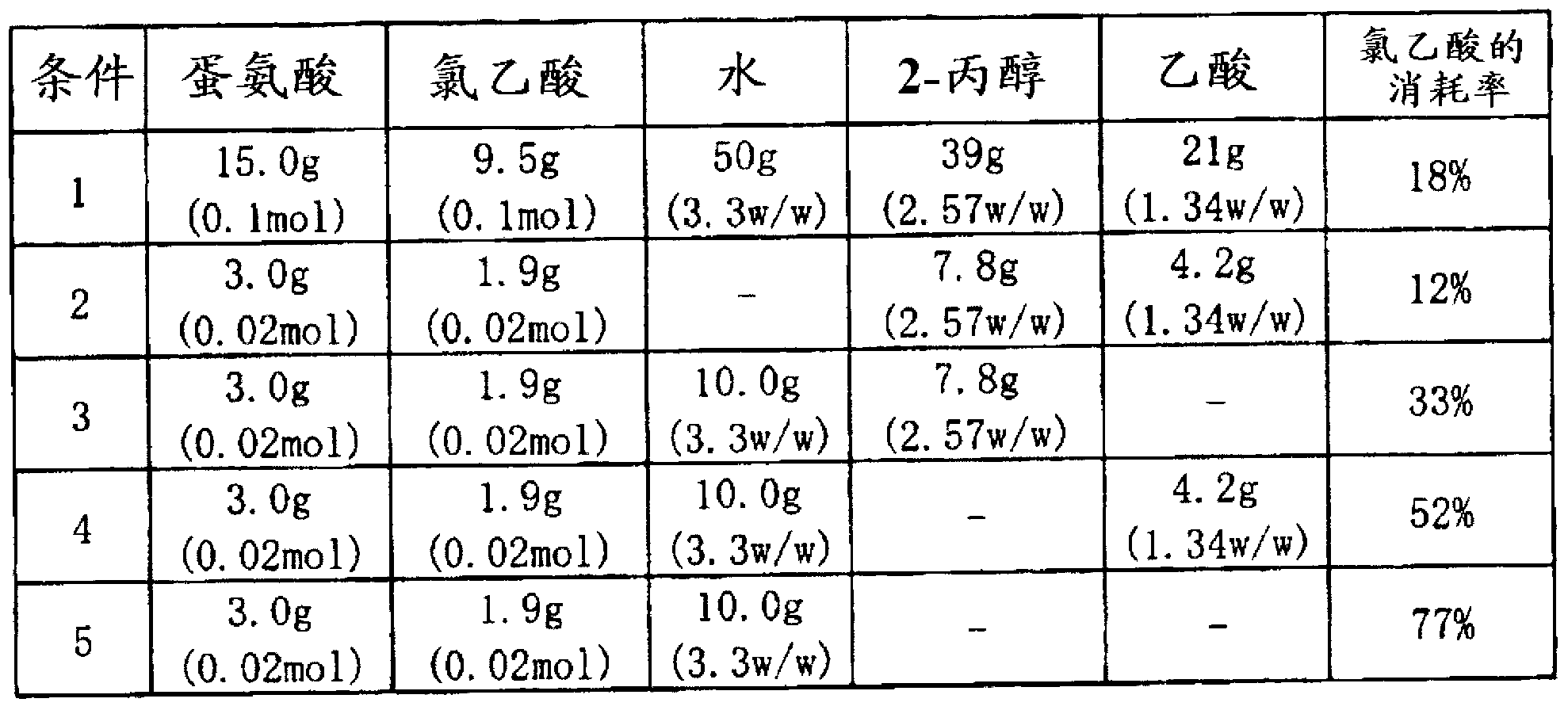
Method used
Image
Examples
Embodiment 1
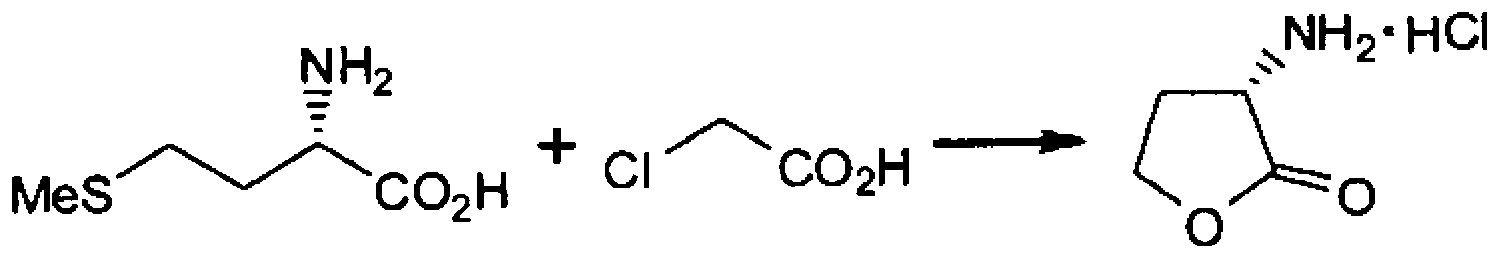
[0028] Example 1 (production of α-amino-γ-butyrolactone hydrochloride)
[0029]
[0030] While stirring the mixture of L-methionine (100.0g, 0.67mol) and water (200ml), after heating up to 81°C, add monochloroacetic acid (63.3g, 0.67mol) dropwise in water ( 100ml) solution. The reaction mixture was further stirred at this temperature for about 3 hours. After cooling the reaction mixture to 25° C., it was washed with ethyl acetate (200 ml×2 times, 100 ml×3 times) to obtain an aqueous layer (about 393 g). Among them, 79.9g of the water layer (equivalent to methionine 0.13mol) was used, concentrated to a residual amount of about 29g under the condition of 50-60°C / about 5KPa, and 35% hydrochloric acid (14.0g, 0.13mol), stirred for about 0.5 hours. After cooling to 25°C, 2-propanol (100ml) was added dropwise, followed by further cooling to 0-5°C. Filtration, washing with 40 ml of 2-propanol, and drying under reduced pressure gave 12.6 g of α-amino-γ-butyrolactone hydrochlori...
Embodiment 2
[0031] Example 2 (production of α-amino-γ-butyrolactone hydrochloride)
[0032] While stirring the mixture of L-methionine (200g, 1.34mol) and water (266ml), while raising the temperature to 85°C, add monochloroacetic acid (127g, 1.34mol) dropwise in water (200ml) at 85-100°C for 2 hours solution. The reaction mixture was further stirred at 85-95°C for about 3 hours. After cooling the reaction mixture to 25° C., it was washed with ethyl acetate (400 ml×1 time, 200 ml×2 times) to obtain a water layer (about 665 g). Among them, 453 g of the aqueous layer (equivalent to methionine 136 g, 0.91 mol) was used, concentrated to a residual amount of about 251 g under the condition of about 55 ° C / about 5 KPa, and hydrogen chloride was dissolved in about 5 hours while stirring at about 50 ° C. Gas (110 g, 3.02 mol) was bubbled into the concentrate. After cooling to 5-10° C., the solid was filtered and washed with 2-propanol (140 ml). Drying under reduced pressure gave 95.2 g of α-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com