Nitroimidazole energetic compound substituted by polynitrobenzene and preparation method thereof
The technology of nitroimidazole and compound is applied in the field of nitroimidazole energetic compound substituted by polynitrobenzene of novel high-energy insensitive explosive and its preparation field, and achieves the effects of simple reaction steps, little environmental harm, and safe and reliable production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
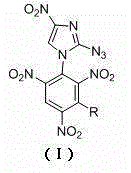
[0025] The preparation method of the 2-azido-4-nitroimidazole substituted by polynitrobenzene of the present invention comprises the following steps:
[0026] In the first step, dissolve 2-azido-4-nitroimidazole in isopropanol, add base and tetrabutylammonium bromide at room temperature, and stir for 0.5h.
[0027] In the second step, the substituted trinitrochlorobenzene is added to the above reaction solution and reacted at 75~85°C. After the reaction is completed, it is cooled to room temperature, and the mixed solution is filtered, washed with methanol, washed with water, and dried to obtain the compound (I).
[0028] Wherein, in the first step, the mol ratio of 2-azido-4-nitroimidazole and alkali is 1:1, and the mol ratio of 2-azido-4-nitroimidazole and tetrabutylammonium bromide is 10 :1~20:1, among which KHCO is selected as the base 3 、K 2 CO 3 Or KOH, the reaction time is 0.5~1h.
[0029] The substituted trinitrochlorobenzene in the second step is 2,4,6-trinitroch...
Embodiment 1
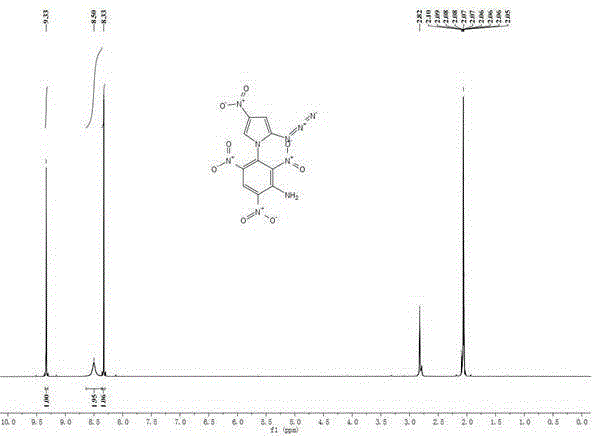
[0032] Add 0.15g (1.0mmol) of 2-azido-4-nitroimidazole to 15mL of isopropanol, add 0.10g (1mmol) of potassium bicarbonate and 0.03g (0.1mmol) of tetrabutyl bromide under stirring at room temperature Ammonium, stirred at room temperature for 0.5h. 0.25 g (1.0 mmol) of 2,4,6-trinitrochlorobenzene was added thereto, and the temperature was raised to 85° C. for 4 h. Cool to room temperature, filter, wash with 5 mL of methanol, and wash with a large amount of water to obtain a yellow solid 1-(2',4',6'-trinitrobenzene)-2-azido-4-nitroimidazole with a yield of 68%.
[0033] m.p.99~101℃;
[0034] 1 HNMR (Acetone- d 6 ,500MHz):8.43(s,1H),9.46(s,2H);
[0035] Elemental Analysis: C 9 h 3 N 9 o 8 , Calculated value: C, 29.60; H, 0.83; N, 34.52; Measured value: C, 29.63; H, 0.81; N, 34.51;
[0036] The compound has both nitroimidazole and polynitrobenzene structures, and has potential applications in the field of high-energy insensitive energetic materials. The theoretically calc...
Embodiment 2
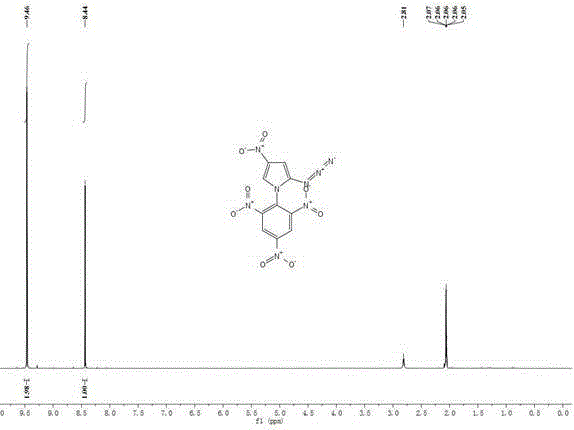
[0038] Add 0.15g (1.0mmol) of 2-azido-4-nitroimidazole to 15mL of isopropanol, add 0.10g (1mmol) of potassium bicarbonate and 0.015g (0.05mmol) of tetrabutyl bromide under stirring at room temperature Ammonium, stirred at room temperature for 0.5h. 0.22g (0.9mmol) of 2,4,6-trinitrochlorobenzene was added thereto, and the temperature was raised to 85°C for 6h. Cool to room temperature, filter, wash with 5 mL of methanol, and wash with a large amount of water to obtain a yellow solid with a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com