A kind of pyridofuroxan energetic compound and its preparation method
A technology of furoxan and compound, which is applied in organic chemistry and other fields, can solve the problems that the detonation performance of pyridine ring series energetic compounds cannot be fully utilized, and achieve the effects of small environmental hazards, mild conditions, and safe and reliable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
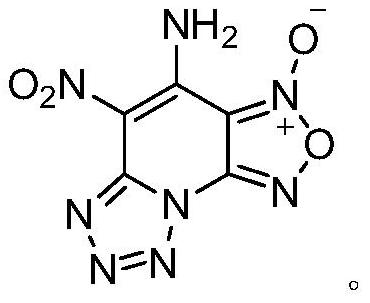
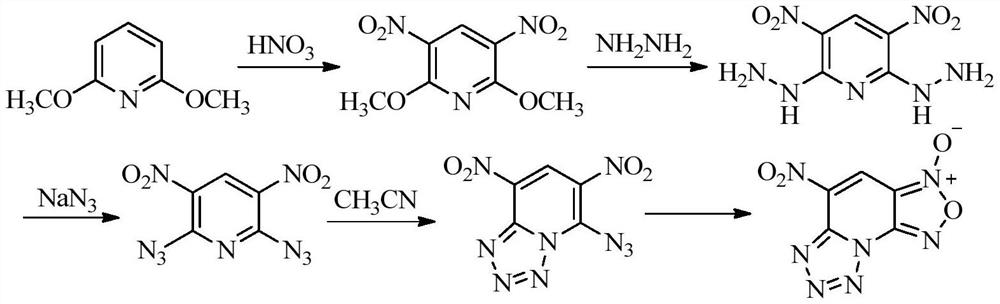
[0024] The preparation method of 4-amino-5-nitro-[1,2,5]oxadiazolo[3,4-e]tetrazol[1,5-a]pyridine-3-oxide of the present invention comprises the following steps :
[0025] In the first step, 4-amino-2,6-dichloro-3,5-dinitropyridine was dissolved in a suitable solvent, sodium azide was added at room temperature, and the reaction was stirred for 1 hour;
[0026] In the second step, a small amount of concentrated hydrochloric acid is added to the reaction mixture, and the temperature is raised to react for a period of time. After the reaction is completed, the mixture is evaporated under reduced pressure to remove the solvent, dissolved in water, extracted with ethyl acetate, dried on the organic phase, and distilled under reduced pressure to obtain 4-Amino-5-nitro-[1,2,5]oxadiazo[3,4-e]tetrazol[1,5-a]pyridine-3-oxide.
[0027] Wherein, in the first step, the molar ratio of 4-amino-2,6-dichloro-3,5-dinitropyridine to sodium azide is 1:2-1:4, which is used to dissolve 4-amino-2 ,...
Embodiment 1
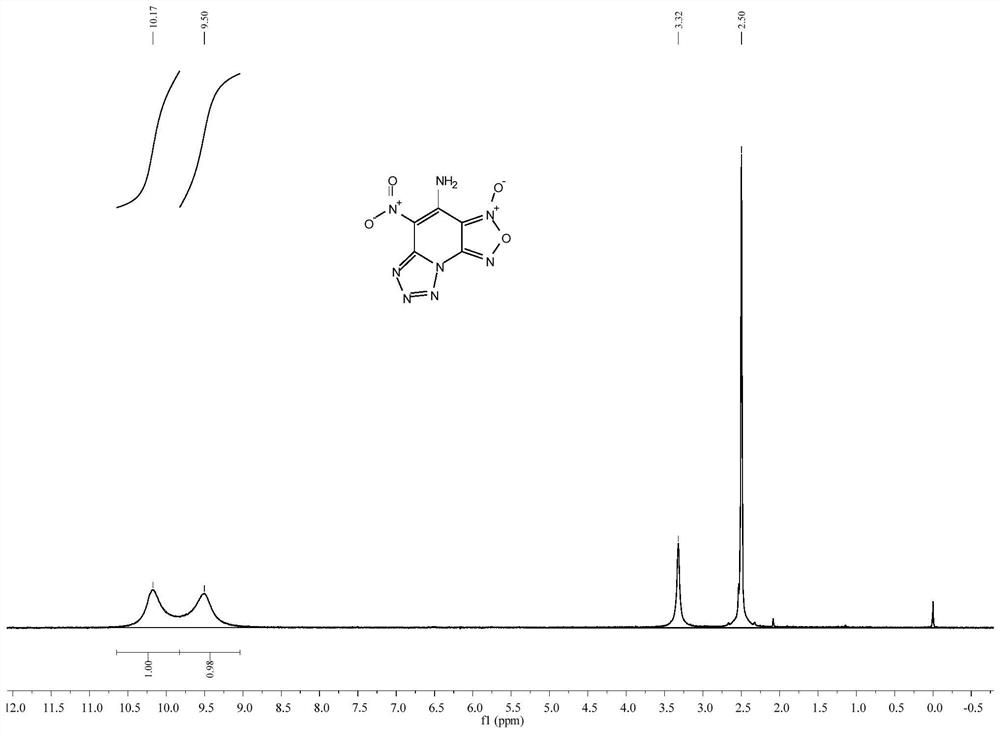
[0030] Dissolve 1.26g (5mmol) of 4-amino-2,6-dichloro-3,5-dinitropyridine in 15mL of acetonitrile, add 0.65g (10mmol) of sodium azide under stirring at room temperature, and stir for 30min. 1 drop of concentrated hydrochloric acid was added to the reaction mixture, and the temperature was raised to 30° C. for 3 h. After the reaction, the mixed solution was rotary evaporated under reduced pressure, dissolved in water, extracted with ethyl acetate, the organic phase was dried and filtered, and the filtrate was distilled under reduced pressure to obtain a yellow solid 4-amino-5-nitro-[1,2,5]oxane Oxadiazo[3,4-e]tetrazol[1,5-a]pyridine-3-oxide 0.89 g, yield 75%. m.p.150-151℃(dec.); 1 H NMR (DMSO-d 6 ,400MHz):δ10.17(s,1H),9.50(s,1H); 13 C NMR (DMSO-d 6 ,100MHz): δ148.96, 144.27, 141.87, 111.17, 105.06; MS (ESI) m / z: 239.0 (M+H). See figure 1 .
[0031] The compound has the structures of tetrazole, furazan oxide and aminonitronitropyridine at the same time, and the theoretical...
Embodiment 2
[0033] Dissolve 1.26g (5mmol) of 4-amino-2,6-dichloro-3,5-dinitropyridine in 15mL of ethyl acetate and acetone mixed solvent, add 0.98g (15mmol) of azide under stirring at room temperature Sodium, stirred and reacted for 30min. Add 1 drop of concentrated hydrochloric acid to the reaction mixture, heat up to 35°C for 2.5h. After the reaction, the mixed solution was rotary evaporated under reduced pressure, dissolved in water, extracted with ethyl acetate, the organic phase was dried and filtered, and the filtrate was distilled under reduced pressure to obtain a yellow solid 4-amino-5-nitro-[1,2,5]oxane Oxadiazo[3,4-e]tetrazol[1,5-a]pyridine-3-oxide 0.71 g, yield 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com