Nitroazidoimidazole energetic ionic salt and preparation method thereof
A technology of nitroazidoimidazole and nitroimidazole, applied in the direction of chemical instruments and methods, preparation of organic compounds, nitrated acyclic/alicyclic/heterocyclic amine explosive composition, etc., to achieve simple synthesis reaction steps and production Safe and reliable, less harmful to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
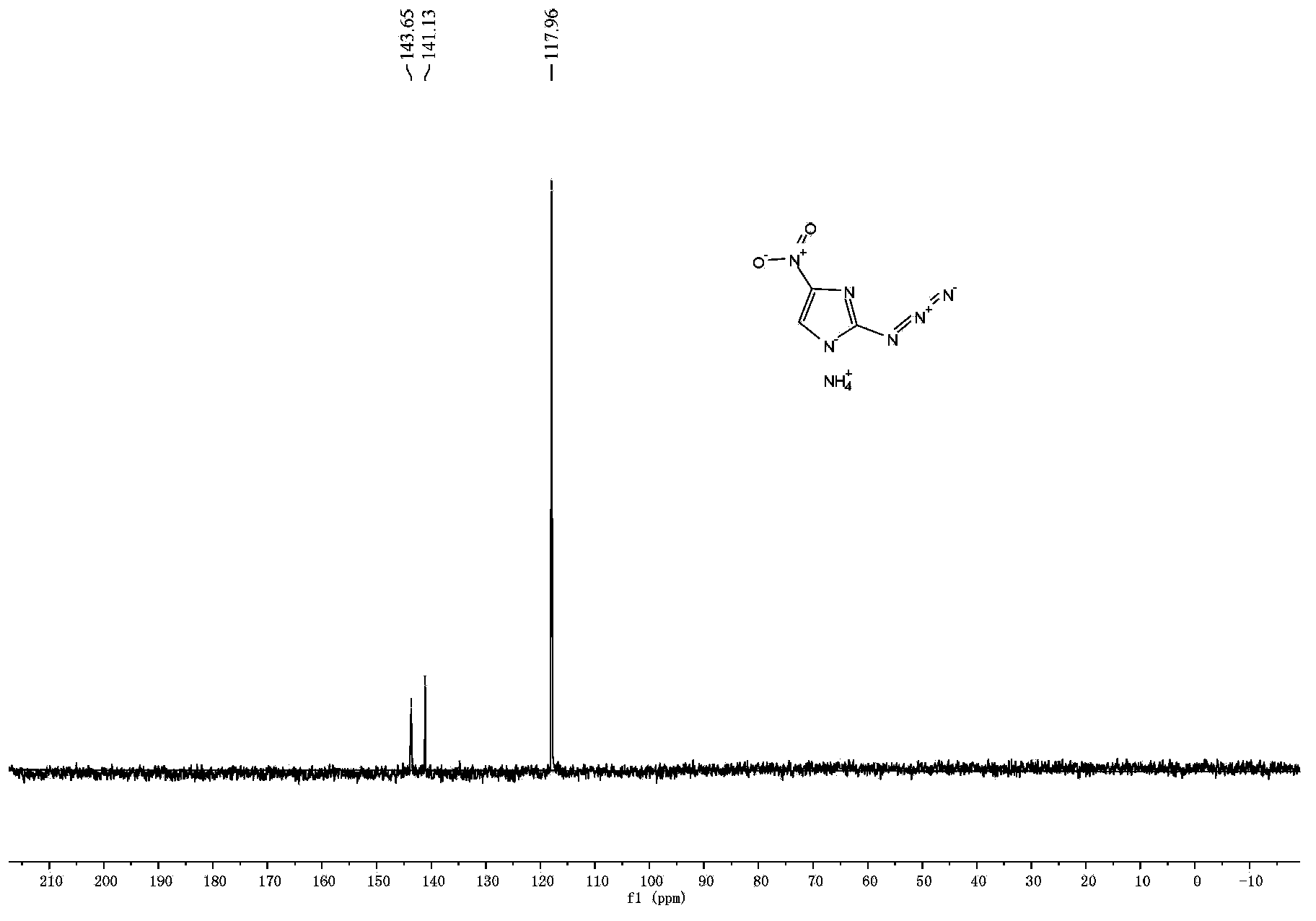
[0030] In an ice-salt bath, add 0.2g of 2-azidoimidazole into 1.4mL of concentrated nitric acid, slowly add 1.4mL of concentrated sulfuric acid dropwise, react at room temperature for 4h, pour into ice water, extract with ethyl acetate, wash the organic phase with saturated brine, Dry over anhydrous sodium sulfate and spin dry to obtain 0.22g, yield 78.6%.
[0031] m.p.141~143℃;
[0032] 1 H NMR (DMSO- d 6 , 500 MHz): 8.27(s, 1H), 13.46(s, 1H);
[0033] Elemental Analysis: C 3 h 2 N 6 o 2 , Calculated: C, 23.38; H, 1.31; N, 54.54; Found: C, 23.26; H, 1.39; N, 54.56;
[0034] MS (ESI) m / z: 152.96 (M-H).
Embodiment 2
[0036] In an ice-salt bath, add 0.2g of 2-azidoimidazole into 2.1mL of concentrated nitric acid, slowly add 2.1mL of concentrated sulfuric acid dropwise, react at room temperature for 4h, pour into ice water, extract with ethyl acetate, wash the organic phase with saturated brine, Dry over anhydrous sodium sulfate and spin dry to obtain 0.23g, yield 82.1%.
Embodiment 3
[0038] Under ice-salt bath, add 0.2g 2-azidoimidazole into 2.0mL concentrated nitric acid, slowly add 3.0mL concentrated sulfuric acid dropwise, react at room temperature for 4h, pour into ice water, extract with ethyl acetate, wash the organic phase with saturated brine, Dry over anhydrous sodium sulfate and spin-dry to obtain 0.24 g with a yield of 85.7%.
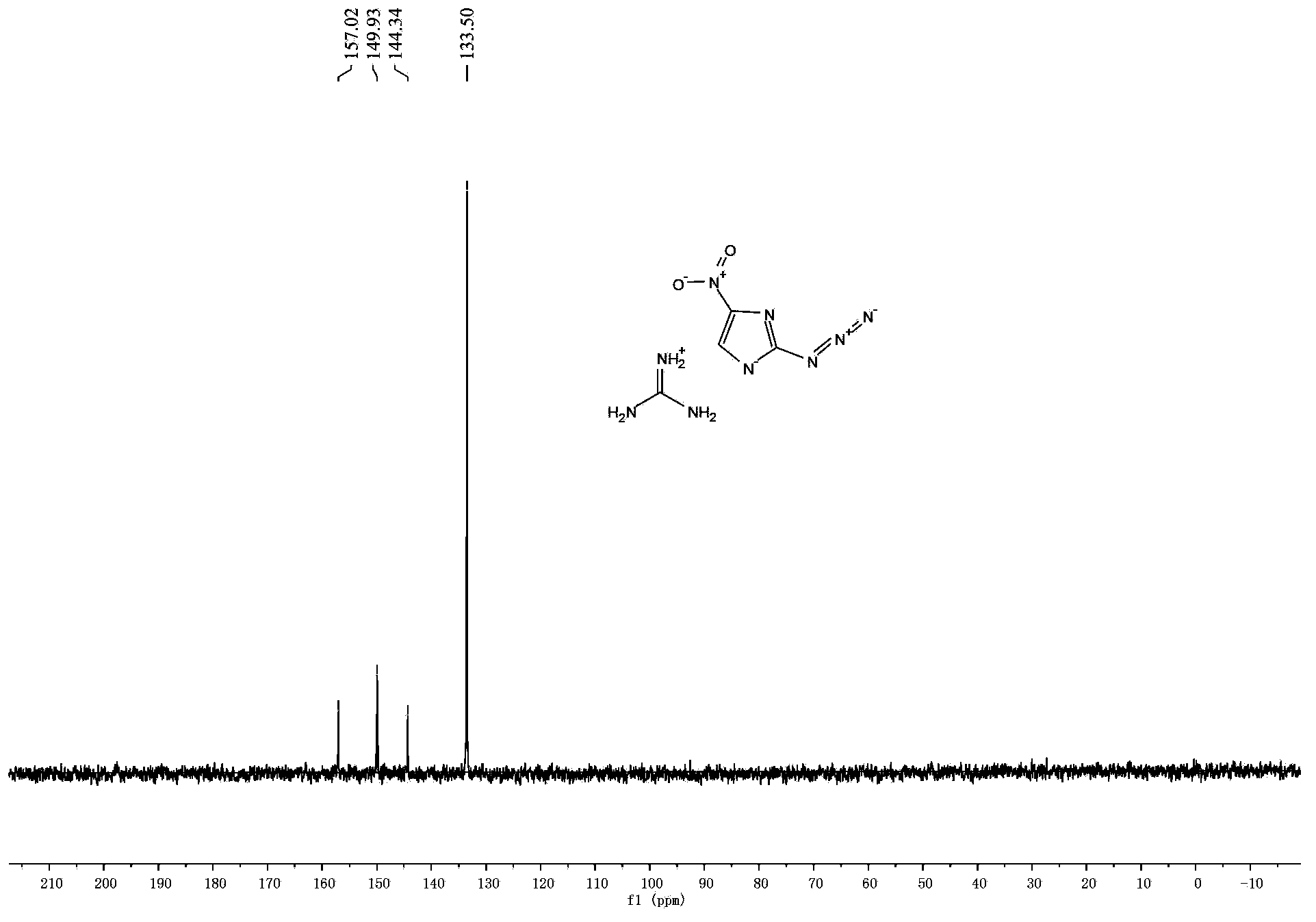
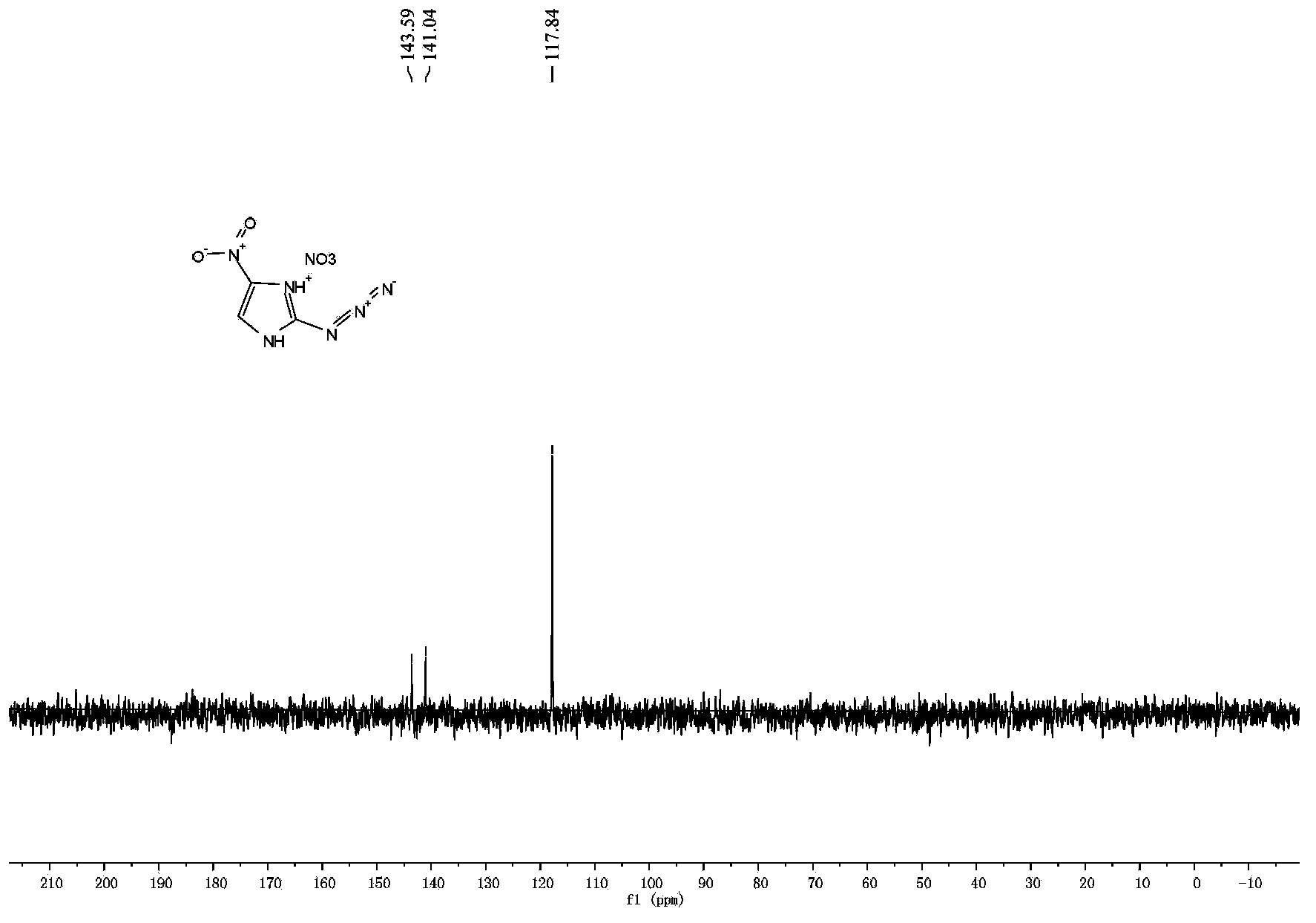
[0039] 2. Synthesis of 2-azido-4-nitroimidazole energetic ion salt
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com